(2S,3S)-1,2-Epoxy-3-(Boc-amino)-4-phenylbutane
MOQ : 1 Kilograms
(2S,3S)-1,2-Epoxy-3-(Boc-amino)-4-phenylbutane Specification
- Molecular Weight
- 263.33 Grams (g)
- Molecular Formula
- C15H21NO3
- Chemical Name
- (2S,3S)-1,2-Epoxy-3-(Boc-amino)-4-phenylbutane
- Purity(%)
- 97%
(2S,3S)-1,2-Epoxy-3-(Boc-amino)-4-phenylbutane Trade Information
- Minimum Order Quantity
- 1 Kilograms
- Payment Terms
- Cash Advance (CA), Cash in Advance (CID)
- Supply Ability
- 500 Kilograms Per Month
- Delivery Time
- 3-4 Week
- Sample Available
- Yes
- Sample Policy
- Sample costs shipping and taxes has to be paid by the buyer
- Main Export Market(s)
- Western Europe, Australia, Eastern Europe, Middle East, Central America, South America, Asia, North America, Africa
- Main Domestic Market
- All India
About (2S,3S)-1,2-Epoxy-3-(Boc-amino)-4-phenylbutane
(2S,3S)-1,2-Epoxy-3-(Boc-amino)-4-phenylbutane Synonym: tert-Butyl [S-(R*,R*)]-()-(1-oxiranyl-2-phenylethyl)carbamate Empirical Formula (Hill Notation): C15H21NO3Molecular Weight: 263.33CAS Number: 98737-29-2 assay 99% optical activity []23/D 7, c =0.6 in methanol mp 125-127C(lit.) Description Application Building block employed in the synthesis of HIV-1 protease inhibitors.[2][1] Employed in synthesizing (hydroxyethyl)urea peptidomimetrics[3] and arylsulfonamides possessing anti-HIV activity.[4] Packaging 1, 5 g in glass bottleFAQs of (2S,3S)-1,2-Epoxy-3-(Boc-amino)-4-phenylbutane:
Q: What is the molecular formula of (2S,3S)-1,2-Epoxy-3-(Boc-amino)-4-phenylbutane?
A: The molecular formula of (2S,3S)-1,2-Epoxy-3-(Boc-amino)-4-phenylbutane is C15H21NO3.Q: What is the molecular weight of this product?
A: The molecular weight of (2S,3S)-1,2-Epoxy-3-(Boc-amino)-4-phenylbutane is 263.33 grams (g).Q: What is the purity of (2S,3S)-1,2-Epoxy-3-(Boc-amino)-4-phenylbutane?
A: The purity of this product is 97%.Q: What is the chemical structure classification of this compound?
A: (2S,3S)-1,2-Epoxy-3-(Boc-amino)-4-phenylbutane contains an epoxy group, a Boc-protected amino group, and a phenyl ring.Q: Is (2S,3S)-1,2-Epoxy-3-(Boc-amino)-4-phenylbutane chiral?
A: Yes, this compound is chiral as it has two stereogenic centers at positions 2 and 3 in its structure.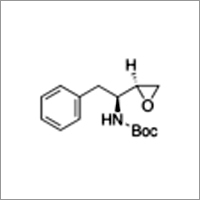
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
More Products in Biochemical & Reagents Category
0.1 M TRIS-HCl pH 8.0 10% PEG 600 solution
Molecular Formula : C4H11NO3 (Tris); H(OCH2CH2)nOH (PEG 600)
Molecular Weight : 121.14 g/mol (Tris); ~600 g/mol (PEG 600)
Melting Point : 171172C (Tris)
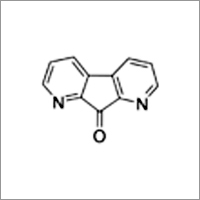
1, 8-Diazafluoren-9-one
Price 100 INR / Kilograms
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C11H6N2O
Molecular Weight : 182.18 GSM (gm/2)
Purity(%) : 99%
Melting Point : 229 to 233 C
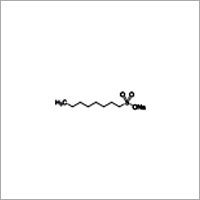
1-Octanesulfonic acid sodium salt
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C8H17NaO3S
Molecular Weight : 216.28
Purity(%) : 98%
Melting Point : 300° C
(2-Carboxyethyl)--cyclodextrin sodium salt
Price 100 INR / Kilograms
Minimum Order Quantity : 1 Kilograms
Molecular Formula : CH2CH2COONa
Molecular Weight : 1414.09 Grams (g)
Purity(%) : 96%

 Send Inquiry
Send Inquiry




 Send Inquiry
Send Inquiry Send SMS
Send SMS Call Me Free
Call Me Free