Actaea racemosa dry extract for system suitability HRS
Actaea racemosa dry extract for system suitability HRS Specification
- Physical Form
- Powder
- Storage
- Store in a cool dry place away from light and moisture
- Purity
- High purity to meet analysis requirements
- Properties
- Contains active compounds characteristic of Actaea racemosa plant extract
- Poisonous
- NO
- Usage
- Used for system suitability testing in pharmaceutical preparations
- Grade
- Pharmaceutical grade
- Shelf Life
- Up to 2 years when stored properly
- Appearance
- Fine brown powder
- Smell
- Herbal
- Application
- Pharmaceutical analysis quality control
- Solubility
- Slightly soluble in water more soluble in ethanol
- Ingredients
- Actaea racemosa plant extract in standardized dry form
- Taste
- Bitter
About Actaea racemosa dry extract for system suitability HRS
Actaea racemosa dry extract for system suitability HRS is a high-purity, pharmaceutical-grade powder formulated for system suitability testing in pharmaceutical preparations. Derived from standardized Actaea racemosa plant extract, it contains active compounds characteristic of the plant to ensure analytical reliability in pharmaceutical analysis and quality control processes. With its fine brown appearance, this product features a distinct herbal smell and a bitter taste, emblematic of its natural origins. It is slightly soluble in water but demonstrates greater solubility in ethanol, providing adaptability across various analytical requirements. Designed to maintain quality, the extract has a shelf life of up to two years when stored in a cool, dry place, protected from light and moisture. Its safety is guaranteed as it is non-poisonous, making it a dependable choice for laboratories requiring consistent and precise results in pharmaceutical applications.
FAQs of Actaea racemosa dry extract for system suitability HRS:
Q: What is the recommended storage condition for this product?
A: Store in a cool, dry place away from light and moisture to ensure optimal quality and shelf life.Q: What is the physical form and appearance of the product?
A: It is a fine brown powder with a herbal smell.Q: What is the solubility of Actaea racemosa dry extract?
A: The product is slightly soluble in water and more soluble in ethanol.Q: How long does the products shelf life last?
A: Its shelf life is up to two years when stored as recommended.Q: Is this product safe for use in pharmaceutical applications?
A: Yes, the product is non-poisonous and meets high-purity standards for system suitability testing.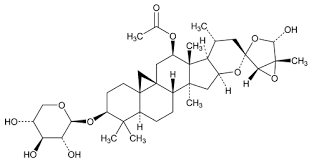
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
More Products in Analytical Grade Chemicals Category
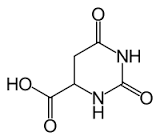
DL-3-Ureidoisobutyric acid
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C5H10N2O3
Molecular Weight : 146.146 Grams (g)
Purity : 96%
Melting Point : 177…178 °C
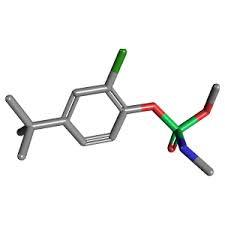
Crufomate
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C12H19ClNO3P
Molecular Weight : 291.71 Grams (g)
Purity : 96%
Melting Point : 61.5°C.
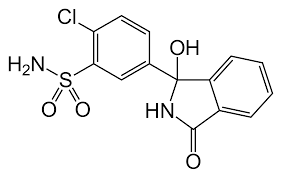
Altizide impurity B
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C11H14ClN3O4S3
Molecular Weight : 383.89456 Grams (g)
Melting Point : >199°C
Bauxite
Price 100 INR
Minimum Order Quantity : 1 , , Kilograms
Molecular Formula : Al2H2O4
Molecular Weight : 119.977 Grams (g)
Purity : 98%
Melting Point : 30°C

 Send Inquiry
Send Inquiry



 Send Inquiry
Send Inquiry Send SMS
Send SMS Call Me Free
Call Me Free