Ammonium chloride
MOQ : 1 Kilograms
Ammonium chloride Specification
- Usage
- Ammonium chloride is used as a flux in preparing metals to be tin coated, galvanized, or soldered. It is an expectorant in cough medicine and as a systemic acidifying agent in the treatment of severe metabolic alkalosis.
- Molecular Weight
- 53.491 Grams (g)
- Solubility
- Ammonia, Water, Methanol, Alcohol, Glycerol, Hydrazine
- Melting Point
- 338 °C
- Molecular Formula
- NH4Cl
Ammonium chloride Trade Information
- Minimum Order Quantity
- 1 Kilograms
- Payment Terms
- Cash in Advance (CID), Cash Advance (CA)
- Supply Ability
- 500 Kilograms Per Month
- Delivery Time
- 3-4 Week
- Sample Available
- Yes
- Sample Policy
- Sample costs shipping and taxes has to be paid by the buyer
- Main Export Market(s)
- Western Europe, Australia, Middle East, Central America, South America, Asia, Eastern Europe, North America, Africa
- Main Domestic Market
- All India
About Ammonium chloride
Ammonium chloride Synonym: Ammonium chloride, SalmiacLinear Formula: NH4ClMolecular Weight: 53.49CAS Number: 12125-02-9grade certified reference material vapor density 1.9 (vs air) vapor pressure 1 mmHg ( 160.4 C) form neat mp 340C (subl.)(lit.) format neat pharmacopeia traceability traceable to USP 1029953FAQs of Ammonium chloride:
Q: What are the primary uses of Ammonium chloride?
A: Ammonium chloride is used as a flux in preparing metals to be tin-coated, galvanized, or soldered. It is also used as an expectorant in cough medicine and as a systemic acidifying agent in the treatment of severe metabolic alkalosis.Q: What is the molecular formula and weight of Ammonium chloride?
A: The molecular formula of Ammonium chloride is NH4Cl, and its molecular weight is 53.491 grams (g).Q: What is the melting point of Ammonium chloride?
A: The melting point of Ammonium chloride is 338 C.Q: In which substances is Ammonium chloride soluble?
A: Ammonium chloride is soluble in ammonia, water, methanol, alcohol, glycerol, and hydrazine.Q: Can Ammonium chloride be used in metal preparation processes?
A: Yes, Ammonium chloride is used as a flux in preparing metals to be tin-coated, galvanized, or soldered.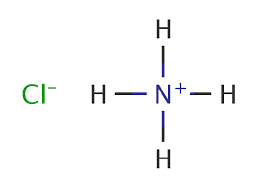
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
More Products in Analytical Grade Chemicals Category
Dimethyltin dichloride
Price 100 INR / Kilograms
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C2H6Cl2Sn
Purity : 99.9%
Vinpocetine
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C22H26N2O2
Melting Point : 147153 °C dec.
Molecular Weight : 350.462 Grams (g)
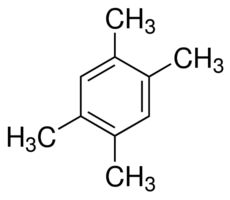
1,2,4,5-Tetramethylbenzene
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C10H14
Melting Point : 79.2 °C
Molecular Weight : 134.22 Grams (g)
Purity : 99%
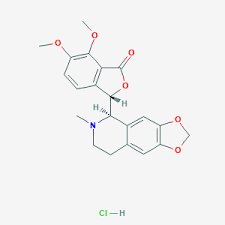
Hydrastine hydrochloride
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C21H21NO6
Melting Point : 132°
Molecular Weight : 383.4 Grams (g)

 Send Inquiry
Send Inquiry
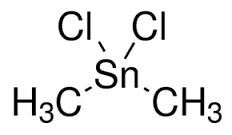


 Send Inquiry
Send Inquiry Send SMS
Send SMS Call Me Free
Call Me Free