Beclometasone dipropionate impurities F and N
Beclometasone dipropionate impurities F and N Specification
- Purity
- 97%
- Melting Point
- 210 C
- Usage
- Beclomethasone is used to prevent and control symptoms (wheezing and shortness of breath) caused by asthma. This medication belongs to a class of drugs known as corticosteroids. It works by reducing the swelling of the airways in the lungs to make breathing easier.
- Molecular Weight
- 521 Grams (g)
- Appearance
- White crystalline powder
Beclometasone dipropionate impurities F and N Trade Information
- Minimum Order Quantity
- 1 Kilograms
- Payment Terms
- Cash in Advance (CID), Cash Advance (CA)
- Supply Ability
- 500 Kilograms Per Month
- Delivery Time
- 3-4 Week
- Sample Available
- Yes
- Sample Policy
- Sample costs shipping and taxes has to be paid by the buyer
- Main Export Market(s)
- Australia, Middle East, Central America, Eastern Europe, South America, Western Europe, Asia, North America, Africa
- Main Domestic Market
- All India
About Beclometasone dipropionate impurities F and N
FAQs of Beclometasone dipropionate impurities F and N:
Q: What is the appearance of Beclometasone dipropionate impurities F and N?
A: It is a white crystalline powder.Q: What is the purity of Beclometasone dipropionate impurities F and N?
A: The purity level is 97%.Q: What is the molecular weight of this product?
A: The molecular weight is 521 grams.Q: What is the melting point of Beclometasone dipropionate impurities F and N?
A: The melting point is 210C.Q: Why are these impurities used in pharmaceutical research?
A: They are used for quality control and to maintain product standards in Beclomethasone formulations.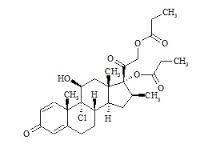

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Analytical Grade Chemicals Category
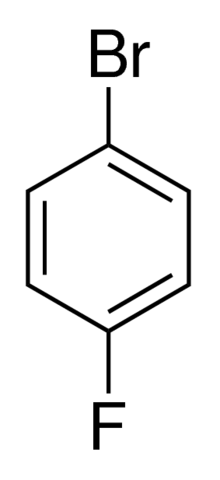
1-Bromo-4-fluorobenzene
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C6H4BrF
Purity : 95%
Appearance : Clear, slightly yellow to clear liquid
Molecular Weight : 175.000 Grams (g)
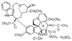
Vincristine sulfate
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C46H58N4O14S
Purity : 96%
Molecular Weight : 923 Grams (g)
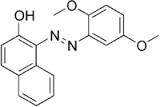
Citrus Red 2
Price 100 INR
Minimum Order Quantity : 1 , , Kilograms
Molecular Formula : C18H16N2O3
Purity : 99%
Appearance : Orange to yellow solid or a dark red powder
Flubenzimin
Price 100 INR
Minimum Order Quantity : 1 , , Kilograms
Molecular Formula : C17H10F6N4S
Purity : 98%
Appearance : Solid powder.
Molecular Weight : 416.3 Grams (g)

 Send Inquiry
Send Inquiry


 Send Inquiry
Send Inquiry Send SMS
Send SMS Call Me Free
Call Me Free