Carbidopa impurity mixture
Carbidopa impurity mixture Specification
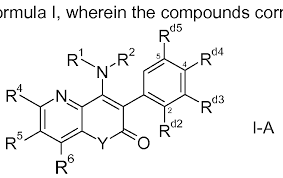
- Structural Formula
- Varies based on specific impurities
- CAS No
- Varies based on specific impurities in the mixture
- Purity
- Not applicable (impurity mixture)
- Shelf Life
- Typically 2 years under proper storage conditions
- Usage
- Used to identify and quantify impurities in Carbidopa formulations
- Grade
- Pharmaceutical
- Properties
- Pharmaceutical-grade mixture containing trace levels of related impurities
- Poisonous
- YES
- Application
- Pharmaceutical reference standard for quality control and research purposes
- Smell
- Odorless or faint characteristic odor
- Molecular Formula
- Varies based on specific impurities (e.g. C10H14N2O4 for Carbidopa)
- HS Code
- 2942000000
- Physical Form
- Powder
- Solubility
- Varies depending on solvents; partially soluble in water and organic solvents
- Storage
- Store at controlled room temperature (20-25C) in a tightly sealed container protected from light and moisture
- Appearance
- White to off-white powder
- Melting Point
- Varies based on components in the mixture
- Molecular Weight
- Varies based on specific impurities
- Ingredients
- Carbidopa-related impurities (trace levels exact composition varies)
About Carbidopa impurity mixture
Carbidopa impurity mixture is a pharmaceutical-grade powder used as a reference standard for quality control and research purposes in pharmaceutical formulations containing Carbidopa. It contains trace levels of related impurities essential for identifying and quantifying them during analytical testing to ensure product purity and quality. The mixture varies in molecular formula, structural formula, and melting point depending on the specific impurities present. It has an appearance ranging from white to off-white, is odorless or has a faint characteristic odor, and its solubility depends on the solvent, with partial solubility in water and organic solvents. The product must be stored in a controlled room temperature environment (2025C) in a tightly sealed container away from light and moisture to maintain its integrity. Typically, it has a shelf life of two years under proper storage conditions. Note that the formulation is poisonous and should be handled with care by trained professionals only.
FAQs of Carbidopa impurity mixture:
Q: What is the primary application of Carbidopa impurity mixture?
A: It is primarily used for identifying and quantifying impurities in pharmaceutical formulations containing Carbidopa.Q: How should this product be stored?
A: Store at controlled room temperature (2025C) in a tightly sealed container protected from light and moisture.Q: What is the physical form of the Carbidopa impurity mixture?
A: The product comes in powder form with an appearance ranging from white to off-white.Q: Can this product dissolve in water?
A: It is partially soluble in water and organic solvents, with solubility varying depending on the solvent.Q: What is the shelf life of Carbidopa impurity mixture?
A: The product typically has a shelf life of two years under proper storage conditions.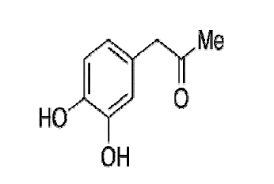
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
More Products in Analytical Grade Chemicals Category
Complex Nutrients - Whole Volume
Molecular Formula : C12H22O11
Purity : >99%
Molecular Weight : 342.30 g/mol
Appearance : Fine white powder
Ellagic acid
Price 100 INR
Minimum Order Quantity : 1 , , Kilograms
Molecular Formula : C14H6O8
Purity : 98%
Molecular Weight : 302.197 Grams (g)
Appearance : cream needles or powder (est)
Bromhexine impurity C
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C14H21Br2ClN2
Purity : 99%
Molecular Weight : 412.59 Grams (g)
Imazalil-(allyl-d5)
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C14H14Cl2N2O
Purity : 98%
Molecular Weight : 297.18 Grams (g)
Appearance : Slightly yellow to brown solidified oil

 Send Inquiry
Send Inquiry


 Send Inquiry
Send Inquiry Send SMS
Send SMS