Cefotaxime for peak identification
Cefotaxime for peak identification Specification
- Appearance
- Off-white to pale yellow crystalline powder
- Poisonous
- No (for analytical and laboratory use only, not for human consumption)
- Usage
- For qualitative and quantitative determination of Cefotaxime peaks in analytical testing
- Purity
- >98%
- Ingredients
- Cefotaxime Sodium
- Storage
- Store at 28C, protected from light and moisture
- Melting Point
- 160-164C (decomposes)
- Taste
- Not for taste assessment (For laboratory/analytical use only)
- Smell
- Odorless or slight characteristic odor
- Molecular Weight
- 477.45 g/mol
- Physical Form
- Powder
- Application
- Used as a reference standard for chromatography and analytical procedures
- Product Type
- Cefotaxime for peak identification
- CAS No
- 63527-52-6
- Molecular Formula
- C16H16N5NaO7S2
- Density
- Gram per cubic centimeter(g/cm3)
- EINECS No
- 264-915-9
- Structural Formula
- Included in the product documentation
- Ph Level
- 4.5-6.5 (1% solution in water)
- Grade
- Analytical
- Shelf Life
- 2 Years from the date of manufacturing when stored properly
- Properties
- Highly pure reference standard for analytical use, stable under recommended storage conditions.
- Shape
- Crystalline powder
- Solubility
- Soluble in water, practically insoluble in acetone and in ether
- HS Code
- 29419090
- Stability
- Stable when stored as recommended
- Transportation
- Ambient
- Traceability
- Certified batch with accompanying CoA
- Intended Use
- Laboratory reagent only, not for drug/household use
- Container Material
- Amber glass, screw cap
- Packaging Type
- Sealed, light-resistant vial
- Risk Phrases
- Not classified as hazardous
- Expiry Date Format
- Stamped on label
Cefotaxime for peak identification Trade Information
- Minimum Order Quantity
- 1 Kilograms
- Payment Terms
- Cash Against Delivery (CAD)
- Supply Ability
- Kilograms
- Delivery Time
- 3-4
- Sample Available
- Yes
- Sample Policy
- Sample costs shipping and taxes has to be paid by the buyer
- Main Export Market(s)
- Asia
- Main Domestic Market
- All India
About Cefotaxime for peak identification
Cefotaxime for peak identification
Synonym: Cefotaxim sodium salt, Cefotaxime sodium salt
-
CAS Number 64485-93-4
-
Empirical Formula (Hill Notation) C16H16N5NaO7S2
-
Molecular Weight 477.45
form neat format neat storage temp. 2-8°C
Analytical Grade Quality
Manufactured with a purity exceeding 98%, this Cefotaxime Sodium reference standard is suitable for rigorous laboratory analysis. Its certified batch and accompanying CoA guarantee traceability, reproducibility, and precise analytical results, making it ideal for peak identification in research or quality control environments.
Safe and Convenient Packaging
The product is securely packaged in a sealed, light-resistant amber glass vial with a screw cap to preserve its stability. Easy to store, the crystalline powder is protected from environmental factors and designed for convenience and safety in laboratory settings.
Usage and Stability Guidance
Optimally stable when stored at 28C and protected from light and moisture, this product retains reliability for up to two years from manufacturing. Each package is stamped with the expiry date, ensuring users have clear shelf-life information.
FAQs of Cefotaxime for peak identification:
Q: How should Cefotaxime Reference Standard be used in analytical procedures?
A: This reference standard is intended exclusively for laboratory applications such as chromatography. It is used for qualitative and quantitative identification of Cefotaxime peaks to ensure method accuracy in analytical and quality control studies.Q: What is the recommended storage condition for this product?
A: The Cefotaxime Reference Standard should be stored at 28C in its original sealed, amber glass vial, sheltered from light and moisture, to maintain its stability and purity throughout its two-year shelf life.Q: When does the Cefotaxime Reference Standard expire, and how is the date indicated?
A: The expiry date is stamped directly on the product label. Under recommended conditions, the product remains stable for two years from the manufacturing date.Q: Where can I find supporting documentation for this reference standard?
A: Each batch is supplied with a Certificate of Analysis (CoA) and includes documentation such as the structural formula to support traceability and quality assurance.Q: What benefits does this product offer for laboratory testing?
A: Researchers benefit from its analysable purity (>98%), stable crystalline form, certified batch traceability, and reliable performance for accurate peak identification in laboratory settings.Q: Is the Cefotaxime Reference Standard hazardous or poisonous?
A: No, it is not classified as hazardous or poisonous. However, it is strictly for analytical use in the laboratory and should not be used for human or animal consumption.Q: What steps should be taken to ensure proper handling and usage of this standard?
A: Always use appropriate laboratory protocols, consult the CoA for batch details, and utilize suitable PPE. Avoid tasting or smelling the powder, and ensure solvents compatible with Cefotaximes solubility are used during analysis.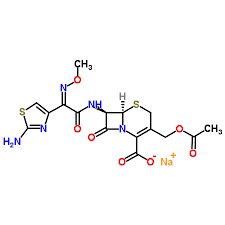

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Analytical Grade Chemicals Category
()-8-THC solution
Molecular Formula : C21H30O2
Molecular Weight : 314.46 g/mol
Purity : 98% or higher
Melting Point : 66 C
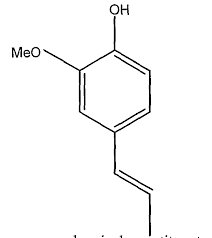
Clove oil
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C7H12ClN3O2
Molecular Weight : 205.64 Grams (g)
Purity : 99%
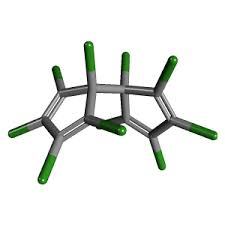
Dienochlor
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C10Cl10
Molecular Weight : 474.6 Grams (g)
Purity : 97.6%
Melting Point : 122–123 °C (252–253 °F; 395–396 K)
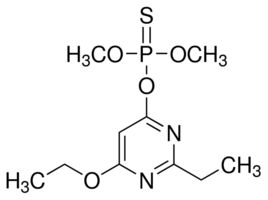
Etrimfos
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C10H17N2O4PS
Molecular Weight : 292.29 Grams (g)
Purity : 98%
Melting Point : (oC), 3.4, L3

 Send Inquiry
Send Inquiry



 Send Inquiry
Send Inquiry Send SMS
Send SMS