Cefotaxime sodium
Cefotaxime sodium Specification
- CAS No
- 64485-93-4
- Product Type
- Active Pharmaceutical Ingredient
- Ph Level
- 4.5-6.5 (in solution)
- Properties
- Broad-spectrum cephalosporin antibiotic, stable under standard conditions, light sensitive
- Structural Formula
- Available on request
- Molecular Weight
- 477.45 g/mol
- Physical Form
- Powder
- Smell
- Odourless
- Shape
- Crystalline powder
- Solubility
- Freely soluble in water
- Ingredients
- Cefotaxime sodium
- Purity
- 98%
- Usage
- Used in intravenous or intramuscular administration
- Molecular Formula
- C16H16N5NaO7S2
- Melting Point
- 178-187C (dec.)
- Taste
- Bitter
- HS Code
- 29419060
- Poisonous
- Non-poisonous as sold
- Appearance
- White to off-white crystalline powder
- Grade
- Pharmaceutical Grade
- Shelf Life
- 3 years
- Storage
- Store in a cool, dry place; protect from light
- Application
- Antibiotic for bacterial infections treatment
- EINECS No
- 264-915-9
- Administration Route
- Parenteral (IV/IM injection)
- Manufacturing Standard
- USP/BP/EP as per requirement
- Therapeutic Class
- Cephalosporin antibiotics
- Bioavailability
- High when administered parenterally
- Impurity Level
- 1.0%
- Colour Reactivity
- Turns slightly yellow on prolonged exposure to air
- Sensitivity
- Sensitive to light, moisture
- Packaging
- Vial, ampoule, or sealed container
- Residual Solvent
- Complies with pharmacopeial standards
- pH Stability
- Stable at pH 4.5-6.5
Cefotaxime sodium Trade Information
- Minimum Order Quantity
- 1 Kilograms
- Payment Terms
- Cash Against Delivery (CAD)
- Supply Ability
- Kilograms
- Delivery Time
- 3-4
- Sample Available
- Yes
- Sample Policy
- Sample costs shipping and taxes has to be paid by the buyer
- Main Export Market(s)
- Asia
- Main Domestic Market
- All India
About Cefotaxime sodium
Cefotaxime sodium
Synonym: Cefotaxim sodium salt, Cefotaxime sodium salt
-
CAS Number 64485-93-4
-
Empirical Formula (Hill Notation) C16H16N5NaO7S2
-
Molecular Weight 477.45
form neat format neat
Effective Broad-Spectrum Antibiotic
Cefotaxime sodium is renowned for its efficacy across a wide range of bacterial strains, targeting both Gram-positive and Gram-negative organisms. Its high bioavailability ensures rapid onset of action when administered via intravenous or intramuscular injection. This makes it a trusted choice in hospital and clinical settings for the treatment of serious infections.
Quality and Stability Assured
Manufactured under stringent USP/BP/EP standards, cefotaxime sodium maintains its pharmaceutical-grade purity (98%) and controlled impurity levels (1.0%). Its stability within a pH range of 4.56.5 ensures consistent clinical performance. Sensitive to environmental factors, it is securely packaged and clearly labeled to maintain its integrity during storage and transport.
FAQs of Cefotaxime sodium:
Q: How should cefotaxime sodium be administered for therapeutic effectiveness?
A: Cefotaxime sodium is administered parenterally through intravenous (IV) or intramuscular (IM) injection. This route ensures high bioavailability and rapid clinical action for treating bacterial infections.Q: What precautions are necessary for storing cefotaxime sodium?
A: Store cefotaxime sodium in a cool, dry place and protect it from light and moisture. Proper storage preserves its potency and extends its shelf life up to three years.Q: When is cefotaxime sodium indicated for use?
A: It is prescribed to treat severe bacterial infections where a broad-spectrum cephalosporin antibiotic is recommended, particularly in hospital or clinical environments.Q: Where is cefotaxime sodium typically packaged for distribution?
A: Cefotaxime sodium is packaged in pharmaceutical vials, ampoules, or sealed containers to preserve stability and prevent exposure to moisture and light.Q: What is the process for ensuring quality and purity in cefotaxime sodium?
A: Manufacturing adheres to USP, BP, or EP standards. Each batch is tested for impurity level (1.0%), residual solvents, and appearance, guaranteeing a minimum purity of 98% before release.Q: What are the benefits of using cefotaxime sodium for infection treatment?
A: This antibiotic exhibits a broad spectrum of activity, high efficacy, and rapid absorption when administered parenterally, making it valuable for combating difficult or severe infections.Q: Is cefotaxime sodium affected by prolonged exposure to air or light?
A: Yes. Prolonged exposure to air may cause a slight yellowish color change, while light and moisture can degrade the powder, so storage in sealed, light-protected containers is essential.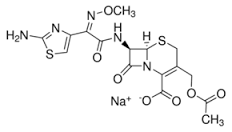

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Analytical Grade Chemicals Category
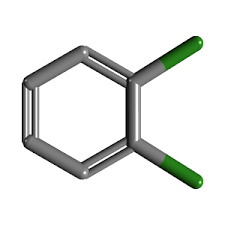
EPA TCLP Base Neutral Dichlorobenzene
Molecular Weight : 147.00 g/mol
Molecular Formula : C6H4Cl2
Purity : Greater than 98%
Melting Point : 52C (12Dichlorobenzene)
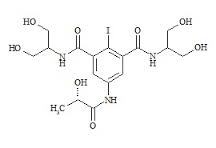
Iopamidol impurity A
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Weight : 777.08 Grams (g)
Molecular Formula : C17H22I3N3O8
Purity : 99%
Melting Point : >3200C (dec)
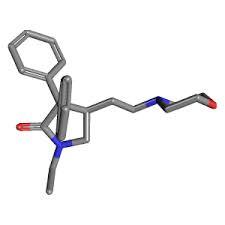
Doxapram hydrochloride
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Weight : 378.507 Grams (g)
Molecular Formula : C24H31ClN2O2
Purity : 98%
Melting Point : (C), 217219
Valproic acid for system suitability
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Weight : 144.211 Grams (g)
Molecular Formula : C8H16O2
Purity : 98%
Melting Point : 300C.

 Send Inquiry
Send Inquiry


 Send Inquiry
Send Inquiry Send SMS
Send SMS Call Me Free
Call Me Free