Chlorinated hydrocarbons on Tenax
Chlorinated hydrocarbons on Tenax Specification
- Solubility
- soluble in most organic solvents
- Usage
- Chlorinated hydrocarbons are widely used as solvents and raw materials for the synthesis of various useful products, such as cleaning agents, pesticides and poly vinyl chloride (PVC). These chlorinated hydrocarbons, however, cause serious environmental problems when they were released into the air or water media.
- Melting Point
- −45 °C (−49 °F; 228 K)
- Molecular Weight
- 96.6 Grams (g)
- Molecular Formula
- C4H13Cl
Chlorinated hydrocarbons on Tenax Trade Information
- Minimum Order Quantity
- 1 Kilograms
- Payment Terms
- Cash in Advance (CID), Cash Advance (CA)
- Supply Ability
- 500 Kilograms Per Month
- Delivery Time
- 3-4 Week
- Sample Available
- Yes
- Sample Policy
- Sample costs shipping and taxes has to be paid by the buyer
- Main Export Market(s)
- Western Europe, Australia, Eastern Europe, Central America, Middle East, South America, Asia, North America, Africa
- Main Domestic Market
- All India
About Chlorinated hydrocarbons on Tenax
FAQs of Chlorinated hydrocarbons on Tenax:
Q: What are the primary applications of chlorinated hydrocarbons on Tenax?
A: They are widely used as solvents and raw materials for producing cleaning agents, pesticides, and PVC.Q: What is the melting point of chlorinated hydrocarbons on Tenax?
A: The melting point is -45 C (-49 F; 228 K).Q: Are chlorinated hydrocarbons soluble in organic solvents?
A: Yes, they are soluble in most organic solvents.Q: What is the molecular formula of chlorinated hydrocarbons?
A: The molecular formula is C4H13Cl.Q: Do chlorinated hydrocarbons pose environmental concerns?
A: Yes, they can cause serious environmental issues if released into air or water. Proper handling and containment are essential.

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Analytical Grade Chemicals Category
Aflatoxin G2
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Weight : ₊.29 Grams (g)
Molecular Formula : C17H14O7
Melting Point : 268.00 °C. @ 760.00 mm Hg (est)
Purity : 98%
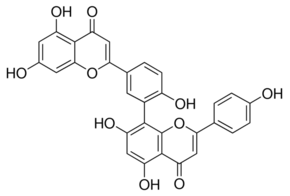
Amentoflavone
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Weight : 538.45 Grams (g)
Molecular Formula : C30H18O10
Purity : 98.5%
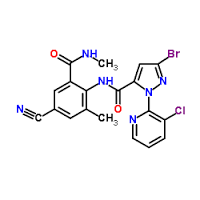
Cyantraniliprole
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Weight : 473.72 Grams (g)
Molecular Formula : C19H14BrClN6O2
Melting Point : 213℃ (dec.)
Purity : 93.4%
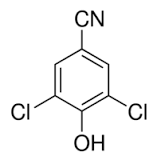
Chloroxynil
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Weight : 188.01 Grams (g)
Molecular Formula : C7H3Cl2NO
Melting Point : 138142°C
Purity : 97%

 Send Inquiry
Send Inquiry



 Send Inquiry
Send Inquiry Send SMS
Send SMS Call Me Free
Call Me Free