Chromium isotopic standard
Chromium isotopic standard Specification
- Usage
- Chromium has four naturally occurring stable isotopes (50Cr, 52Cr, 53Cr, and 54Cr) with natural abundances of 4.35%, 83.79%, 9.50%, and 2.36%, respectively. ... In meteorite samples, the ratio 54Cr/52Cr is sometimes used to report nuclear synthetic anomalies (conventionally reported in epsilon notation).
- Melting Point
- 30 °C
- Molecular Weight
- 51.940505 Grams (g)
- Molecular Formula
- Cr
- Purity
- 97%
Chromium isotopic standard Trade Information
- Minimum Order Quantity
- 1 Kilograms
- Payment Terms
- Cash in Advance (CID), Cash Advance (CA)
- Supply Ability
- 500 Kilograms Per Month
- Delivery Time
- 3-4 Week
- Sample Available
- Yes
- Sample Policy
- Sample costs shipping and taxes has to be paid by the buyer
- Main Export Market(s)
- Western Europe, Africa, Central America, Eastern Europe, Middle East, South America, Asia, North America, Australia
- Main Domestic Market
- All India
About Chromium isotopic standard
FAQs of Chromium isotopic standard:
Q: What is the purity of the Chromium isotopic standard?
A: The Chromium isotopic standard has a purity of 97%.Q: What is the molecular weight of Chromium isotopic standard?
A: The molecular weight of the Chromium isotopic standard is 51.940505 grams.Q: What are the natural abundances of Chromium isotopes in this product?
A: The product includes isotopes with natural abundances of 4.35% (50Cr), 83.79% (52Cr), 9.50% (53Cr), and 2.36% (54Cr).Q: What is the melting point of the Chromium isotopic standard?
A: The melting point of the Chromium isotopic standard is 30 C.Q: How is the isotopic standard utilized in meteorite studies?
A: The ratio 54Cr/52Cr is used to detect nuclear synthetic anomalies, commonly reported in epsilon notation, in meteorite samples.

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Analytical Grade Chemicals Category
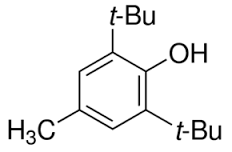
Butylhydroxytoluene Solution
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C15H24O
Appearance : white crystals or crystalline powder
Solubility : Insoluble in water
Molecular Weight : 220.35 Grams (g)
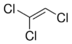
Trichloroethylene
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C2HCl3
Appearance : Colorless liquid
Molecular Weight : 131.4 g Grams (g)
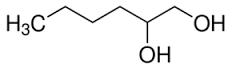
1,2-Hexanediol
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C6H14O2
Appearance : colorless clear liquid (est)
Molecular Weight : 118.17 Grams (g)
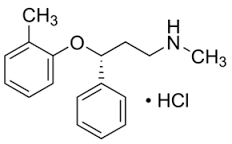
Atomoxetine hydrochloride solution
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C17H22ClNO
Appearance : white solid
Solubility : Soluble in water (27.8mg/mL). DMSO 58 mg/mL. Ethanol 37 mg/mL. Chloroform, methanol.
Molecular Weight : 291.82.

 Send Inquiry
Send Inquiry


 Send Inquiry
Send Inquiry Send SMS
Send SMS Call Me Free
Call Me Free