Ciclesonide impurity C
Ciclesonide impurity C Specification
- Molecular Weight
- 540.7 Grams (g)
- Melting Point
- 60 °C
- Usage
- Ciclesonide is used to prevent and reduce the symptoms (wheezing and shortness of breath) caused by asthma. Controlling asthma symptoms may decrease time lost from work or school. This medication belongs to a class of drugs known as corticosteroids.
- Purity
- 95%
- Molecular Formula
- C32H44O7
Ciclesonide impurity C Trade Information
- Minimum Order Quantity
- 1 Kilograms
- Payment Terms
- Cash in Advance (CID), Cash Advance (CA)
- Supply Ability
- 500 Kilograms Per Month
- Delivery Time
- 3-4 Week
- Sample Available
- Yes
- Sample Policy
- Sample costs shipping and taxes has to be paid by the buyer
- Main Export Market(s)
- Western Europe, Australia, South America, Eastern Europe, Middle East, Central America, Asia, North America, Africa
- Main Domestic Market
- All India
About Ciclesonide impurity C
FAQs of Ciclesonide impurity C:
Q: What is the purity level of Ciclesonide Impurity C?
A: The purity level of Ciclesonide Impurity C is 95%.Q: What is the molecular formula of Ciclesonide Impurity C?
A: The molecular formula of Ciclesonide Impurity C is C32H44O7.Q: What is the melting point of Ciclesonide Impurity C?
A: The melting point of Ciclesonide Impurity C is 60C.Q: What is the molecular weight of Ciclesonide Impurity C?
A: The molecular weight of Ciclesonide Impurity C is 540.7 g/mol.Q: What is the primary use of Ciclesonide Impurity C?
A: Ciclesonide Impurity C is primarily used for research, quality control, and analysis in pharmaceutical applications, especially relating to corticosteroid formulations for asthma treatment.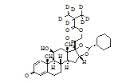

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Analytical Grade Chemicals Category
AOZ
Price 100 INR
Minimum Order Quantity : 1 , , Kilograms
Appearance : Orange Yellow Powder
Molecular Formula : C3H5N3O
Molecular Weight : 99.09 g/mol
Purity : >= 99%
exo-THC solution
Appearance : Clear to pale yellow liquid
Molecular Formula : C21H30O2
Molecular Weight : 314.46 g/mol
Purity : 98%
EPA Phase V Volatile Organic Compounds Mix 9
Appearance : Clear to pale liquid
Molecular Formula : Variable; mixture of VOCs
Molecular Weight : Variable; dependent on specific compounds
Purity : High purity; dependent on component specifications
(+)-Valencene
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Appearance : yellow liquid
Molecular Formula : C15H24
Molecular Weight : 204.357 Grams (g)
Purity : 75%

 Send Inquiry
Send Inquiry



 Send Inquiry
Send Inquiry Send SMS
Send SMS