Elemental Impurities according to ICH Q3D oral, Standard 2
Elemental Impurities according to ICH Q3D oral, Standard 2 Specification
- Shelf Life
- Stable when stored as instructed.
- Smell
- Odorless
- Storage
- Store in a clean dry well-ventilated area at room temperature.
- Ingredients
- N/A (Defined as standard testing material composition may depend on supplier).
- Taste
- Neutral
- HS Code
- 382200
- Application
- Used to control and monitor elemental impurities in drug products as per ICH Q3D guidelines.
- Properties
- Regulatory standard material for pharmaceutical impurity analysis.
- Grade
- Pharmaceutical standard grade.
- Ph Level
- Neutral (7.0)
- Purity
- N/A (Reflective of specificity for standards).
- Appearance
- May vary (Powdery or colorless liquid).
- Poisonous
- NO
- Usage
- Pharmaceutical quality control; compliance with regulatory standards.
- Shape
- N/A (Dependent on supplied form).
- Physical Form
- Standards may appear in powder or solution form depending on the supplied material.
About Elemental Impurities according to ICH Q3D oral, Standard 2
Elemental Impurities according to ICH Q3D oral, Standard 2shelf life limited shelf life, nexpiry date on the labelcomposition Au, 100mg/LIr, 100mg/LOs, 100mg/LPd, 100mg/LPt, 100mg/LRh, 100mg/LRu, 100mg/Lconcentration 10% in hydrochloric acidFAQs of Elemental Impurities according to ICH Q3D oral, Standard 2:
Q: What is the main application of Elemental Impurities according to ICH Q3D oral, Standard 2?
A: Elemental Impurities are used to control and monitor elemental impurities in drug products in compliance with ICH Q3D guidelines for pharmaceutical quality control.Q: How should Elemental Impurities be stored?
A: Elemental Impurities should be stored in a clean, dry, well-ventilated area at room temperature.Q: What form does Elemental Impurities typically appear in?
A: Elemental Impurities may appear as a powder or a colorless liquid depending on the supplied material.Q: Is Elemental Impurities poisonous?
A: No, Elemental Impurities are not poisonous.Q: What is the pH level of Elemental Impurities according to ICH Q3D oral, Standard 2?
A: The pH level of Elemental Impurities is neutral, with a value of 7.0.Q: What is the shelf life of Elemental Impurities?
A: Elemental Impurities are stable when stored as instructed.Q: What grade is Elemental Impurities classified under?
A: Elemental Impurities are classified under the pharmaceutical standard grade for regulatory testing.
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
More Products in Analytical Grade Chemicals Category
Trichloroacetonitrile
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C2Cl3N
Molecular Weight : 144.38 Grams (g)
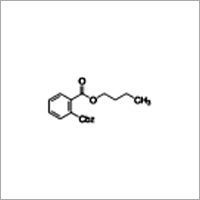
Benzyl butyl phthalate
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C19H20O4
Melting Point : 35 °C (−31 °F; 238 K)
Purity : 99.5%
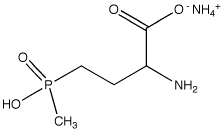
Glufosinate-ammonium
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C5H15N2O4P
Melting Point : 215–218 °C
Purity : 99.5%
Molecular Weight : 198.16 Grams (g)
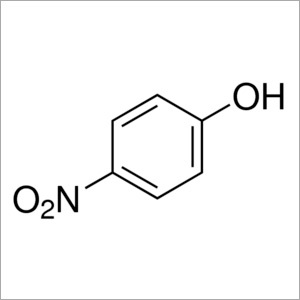
4-Nitrophenol Solution
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C6H5NO3
Melting Point : 113 to 114 C (235 to 237 F; 386 to 387 K)
Purity : 98%
Molecular Weight : 139.110 Grams (g)

 Send Inquiry
Send Inquiry
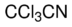


 Send Inquiry
Send Inquiry Send SMS
Send SMS