Ethosuximide impurity A
Ethosuximide impurity A Specification
- Molecular Formula
- C7H11NO2
- Usage
- Ethosuximide is an succinimide based anticonvulsant commonly used for absence (petit mal) seizures in both adults and children. Ethosuximide has been associated with rare instances of serum enzyme elevations during treatment, but has not been linked to cases of clinically apparent liver injury with jaundice.
- Appearance
- White to Off-White Solid
- Melting Point
- 64 to 65 °C (147 to 149 °F)
- Molecular Weight
- 141.17 Grams (g)
Ethosuximide impurity A Trade Information
- Minimum Order Quantity
- 1 Kilograms
- Payment Terms
- Cash Advance (CA), Cash in Advance (CID)
- Supply Ability
- 500 Kilograms kg Per Month
- Delivery Time
- 2-8 Week
- Sample Available
- Yes
- Sample Policy
- Sample costs shipping and taxes has to be paid by the buyer
- Packaging Details
- Carton and Poly Bag.
- Main Export Market(s)
- North America, Eastern Europe, Central America, Africa, Middle East, South America, Western Europe, Australia, Asia
- Main Domestic Market
- All India
About Ethosuximide impurity A
FAQs of Ethosuximide impurity A:
Q: What is the appearance of Ethosuximide Impurity A?
A: Ethosuximide Impurity A appears as a white to off-white solid.Q: What is the melting point of Ethosuximide Impurity A?
A: The melting point ranges between 6465 C (147149 F).Q: What is the molecular formula of this product?
A: The molecular formula is C7H11NO2.Q: How is Ethosuximide generally used?
A: Ethosuximide is commonly used as a succinimide-based anticonvulsant for managing absence seizures in both adults and children.Q: Is Ethosuximide associated with liver-related side effects?
A: Rare instances of serum enzyme elevations during treatment have been reported, but Ethosuximide has not been linked to cases of clinically apparent liver injury with jaundice.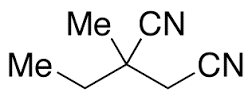

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Analytical Grade Chemicals Category
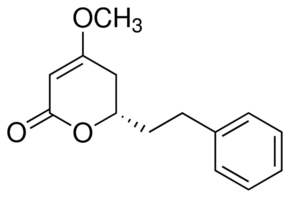
(S)-(+)-7,8-Dihydrokavain
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C14H16O3
Purity : 99.04%
Molecular Weight : 232.27 Grams (g)
Appearance : OffWhite Solid
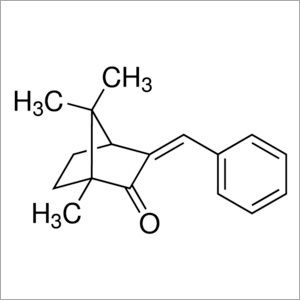
3-Benzylidenecamphor
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C17H20O
Purity : 96%
Molecular Weight : 240.34 Grams (g)
Appearance : Colorless to pale yellow solid
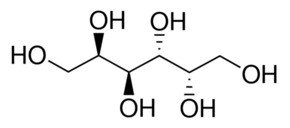
Galactitol Powder
Price 100 INR / Kilograms
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C6H14O6
Purity : 99%
Molecular Weight : 182.172 Grams (g)
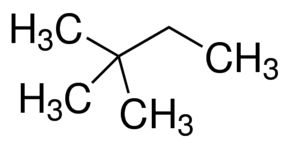
2,2- Dimethylbutane
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C6H14
Purity : 95%
Molecular Weight : 86.178 Grams (g)
Appearance : Colorless liquid

 Send Inquiry
Send Inquiry


 Send Inquiry
Send Inquiry Send SMS
Send SMS