Fosinopril impurity I
MOQ : 1 Kilograms
Fosinopril impurity I Specification
- Purity
- 98%
- Melting Point
- 149-153 °C
- Usage
- Fosinopril is used to treat high blood pressure (hypertension). Lowering high blood pressure helps prevent strokes, heart attacks, and kidney problems. It is also used to treat heart failure. Fosinopril is an ACE inhibitor and works by relaxing blood vessels so that blood can flow more easily.
- Molecular Weight
- 563.663 Grams (g)
- Molecular Formula
- C30H45NNaO7P
Fosinopril impurity I Trade Information
- Minimum Order Quantity
- 1 Kilograms
- Payment Terms
- Cash Advance (CA), Cash in Advance (CID)
- Supply Ability
- 500 Kilograms Per Month
- Delivery Time
- 2-8 Week
- Sample Available
- Yes
- Sample Policy
- Sample costs shipping and taxes has to be paid by the buyer
- Main Export Market(s)
- South America, Central America, Eastern Europe, Africa, Middle East, Western Europe, Australia, Asia, North America
- Main Domestic Market
- All India
About Fosinopril impurity I
Fosinopril impurity I is a high-purity compound with a purity level of 98%, specifically designed for applications in pharmaceutical research and development. With a molecular formula of C30H45NNaO7P and a molecular weight of 563.663 grams, this impurity provides reliable and consistent results in the study of Fosinopril formulations. The product has a melting point range of 149-153C, ensuring its stability under specified conditions. Fosinopril, the primary active ingredient, is widely used in the treatment of hypertension and heart failure as an ACE inhibitor. It works by relaxing blood vessels, improving blood flow, and reducing risks such as strokes, heart attacks, and kidney issues. Fosinopril impurity I serves as an essential material for pharmaceutical businesses, aiding in quality control and compliance testing of Fosinopril-based medications.
FAQs of Fosinopril impurity I:
Q: What is the molecular formula of Fosinopril impurity I?
A: The molecular formula of Fosinopril impurity I is C30H45NNaO7P.Q: What is the purity level of this product?
A: Fosinopril impurity I has a purity level of 98%.Q: What is the melting point of this impurity?
A: The melting point range of Fosinopril impurity I is 149-153C.Q: What is the molecular weight of this product?
A: The molecular weight of Fosinopril impurity I is 563.663 grams.Q: What is the primary usage of Fosinopril?
A: Fosinopril is primarily used to treat high blood pressure (hypertension) and heart failure by relaxing blood vessels for better blood flow.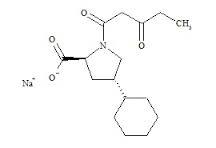
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
More Products in Analytical Grade Chemicals Category
Desbutylbupivacaine
Molecular Formula : C17H26N2O
Purity : 99%
Molecular Weight : 274.4 g/mol
Melting Point : 8890C
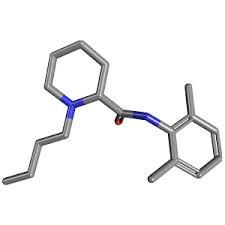
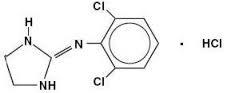
Clonidine hydrochloride
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C9H9Cl2N3
Purity : 99%
Molecular Weight : 230.093 Grams (g)
Melting Point : >300°C
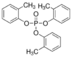
Tri-o-tolyl phosphate
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C21H21O4P
Purity : 97.5%
Molecular Weight : 368.4 Grams (g)
Melting Point : 40 °C (−40 °F; 233 K)
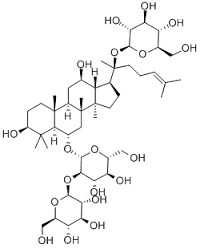
Ginsenoside Rd
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C48H82O18
Purity : 98%
Molecular Weight : 947.166 Grams (g)

 Send Inquiry
Send Inquiry


 Send Inquiry
Send Inquiry Send SMS
Send SMS