Glacial Acetic Acid
Glacial Acetic Acid Specification
- Molecular Weight
- 60.052 Grams (g)
- Melting Point
- 16.6 °C
- Appearance
- Colourless crystalline solid.
- Usage
- It is called glacial because it solidifies just less than room temp at 16.7 degrees C. Acetic acid is used extensively as a chemical reagent particularly to produce cellulose acetate for photographic film. It is also used in the production of polyvinylacetate for wood glue. A 3–9% solution is used in making vinegar.
- Molecular Formula
- CH3COOH
Glacial Acetic Acid Trade Information
- Minimum Order Quantity
- 1 Kilograms
- Payment Terms
- Cash Advance (CA), Cash in Advance (CID)
- Supply Ability
- 500 Kilograms KG Per Month
- Delivery Time
- 2-8 Days
- Sample Available
- Yes
- Sample Policy
- Sample costs shipping and taxes has to be paid by the buyer
- Packaging Details
- Carton and Poly Bag.
- Main Export Market(s)
- Western Europe, Australia, North America, South America, Central America, Africa, Asia
- Main Domestic Market
- All India
About Glacial Acetic Acid
Glacial Acetic AcidPharmaceutical Secondary Standard;Synonym: Acetic acid, Glacial acetic acidCAS Number64-19-7Linear FormulaCH3CO2HMolecular Weight60.05PropertiesRelated CategoriesAdditional Standards,Analytical Standards,Analytical/Chromatography,Chromatography,Pharmaceutical Secondary Standards,Pharmaceutical and Drug Standards,Pharmacopeia & Metrological Institutes Standards,Secondary Pharmaceutical Standardspharmaceutical secondary standardvapor density 2.07 (vs air)InChI Key QTBSBXVTEAMEQO-UHFFFAOYSA-Nautoignition temp. 800Fexpl. lim. 16%, 92F4%, 59Fpackaging ampule of 3x1.5mLrefractive index n20/D1.371(lit.)bp 117-118C(lit.)mp 16.2C(lit.)density 1.049g/mLat 25C(lit.)FAQs of Glacial Acetic Acid:
Q: What is the molecular formula and molecular weight of Glacial Acetic Acid?
A: The molecular formula is CH3COOH, and the molecular weight is 60.052 grams (g).Q: Why is it called Glacial Acetic Acid?
A: It is called glacial because it solidifies at temperatures just below room temperature (16.7 C) into a colourless crystalline solid.Q: What is the melting point of Glacial Acetic Acid?
A: The melting point of Glacial Acetic Acid is 16.6 C.Q: What is the appearance of Glacial Acetic Acid?
A: Glacial Acetic Acid appears as a colourless crystalline solid.Q: What are the common industrial applications of Glacial Acetic Acid?
A: Glacial Acetic Acid is extensively used as a chemical reagent, particularly for producing cellulose acetate (used in photographic film) and polyvinyl acetate (used in wood glue). Additionally, a 39% solution is used for making vinegar.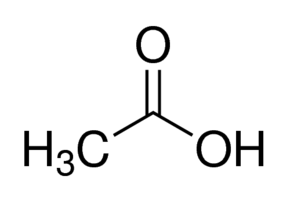

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Analytical Grade Chemicals Category
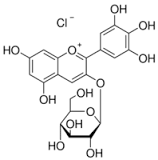
Delphinidin 3-O--D-galactoside chloride
Price 100 INR / Kilograms
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C15H11O7
Molecular Weight : 338.69 Grams (g)
Appearance : Red to dark brown powder
Purity : 99%
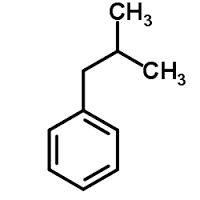
Isobutylbenzene
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C10H14
Molecular Weight : 134.22 Grams (g)
Appearance : Colorless liquid
Purity : 99%
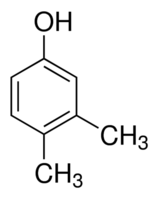
3,4-Dimethylphenol
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C8H10O
Molecular Weight : 122.1644 Grams (g)
Appearance : Colorless crystalline solid
Purity : 98%
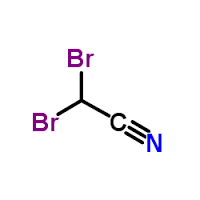
Dibromoacetonitrile (C2HBr2N)
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C2HBr2N
Molecular Weight : 198.84 Grams (g)
Appearance : Clear colorless to yellow liquid
Purity : 92%

 Send Inquiry
Send Inquiry


 Send Inquiry
Send Inquiry Send SMS
Send SMS Call Me Free
Call Me Free