Human coagulation factor VII concentrate BRP
Human coagulation factor VII concentrate BRP Specification
- Solubility
- Soluble in water / physiological saline upon reconstitution
- Storage
- Store at –20°C or below
- Grade
- Pharmaceutical (BRP)
- Smell
- Odorless
- CAS No
- 15676-36-9
- Appearance
- White to off-white powder
- Application
- Laboratory reference standard, coagulation studies, clinical diagnostics
- Usage
- Used for testing and calibration in factor VII assays
- Melting Point
- Not applicable (decomposes)
- Shape
- Fine powder
- Structural Formula
- Protein structure (not applicable for small molecule formula)
- HS Code
- 30029090
- Ph Level
- Neutral (7.0 ± 0.5 after reconstitution)
- Physical Form
- Lyophilized powder
- Properties
- Stable when stored at recommended conditions, high purity, sterile
- Poisonous
- Non-poisonous under proper usage
- Product Type
- Biological Reference Preparation (BRP), Human Plasma Derived
- Molecular Formula
- C3267H5032N882O1002S68 (protein composition)
- Molecular Weight
- ~50 kDa (approximate)
- EINECS No
- 239-791-7
- Shelf Life
- Up to 5 years if unopened and stored correctly
- Purity
- >99%
- Ingredients
- Human coagulation factor VII
- Packing Type
- Glass or plastic vial
- Expiry date indication
- Labeled on each vial
- Quality Standard
- Complies with European Pharmacopeia requirements
- Sterility
- Sterile
- Reconstitution
- Reconstitute with provided buffer or sterile water
- Allergen Notification
- Contains human derived proteins; handle accordingly
- Biological Origin
- Derived from human plasma
- Route of Administration
- Not intended for direct clinical use
Human coagulation factor VII concentrate BRP Trade Information
- Minimum Order Quantity
- 1 Kilograms
- Payment Terms
- Cash in Advance (CID), Cash Advance (CA)
- Supply Ability
- 500 Kilograms Per Month
- Delivery Time
- 2-8 Week
- Sample Available
- Yes
- Sample Policy
- Sample costs shipping and taxes has to be paid by the buyer
- Main Export Market(s)
- Eastern Europe, Western Europe, Middle East, Central America, Africa, South America, Australia, Asia, North America
- Main Domestic Market
- All India
About Human coagulation factor VII concentrate BRP
Human coagulation factor VII concentrate BRP
European Pharmacopoeia (EP) Reference Standard
Synonym: Factor VII from human plasma, Proconvertin
CAS Number 9001-25-6
Properties
| Related Categories | Analytical Standards, Analytical/Chromatography, EP Standards, EP Standards G - H, Pharmacopeia & Metrological Institutes Standards |
| form | neat |
| format | neat |
Laboratory Reference Standard
This product is an essential laboratory reference standard for coagulation assays. It provides accurate, consistent results for factor VII determination, crucial for validating diagnostic protocols and calibrating instrument performance in clinical laboratories.
Compliance with Quality Standards
Manufactured under strict pharmaceutical guidelines, the factor VII concentrate adheres to European Pharmacopeia requirements. Each batch undergoes comprehensive testing to ensure sterility, high purity, and identity, giving researchers confidence in every use.
Convenient Packaging and Storage
Supplied in glass or plastic vials containing a fine, odorless, white to off-white lyophilized powder, the products integrity is supported by appropriate labeling and sealed packaging. It offers a long shelf life when stored at or below 20C, ensuring sustained usability in laboratory environments.
FAQs of Human coagulation factor VII concentrate BRP:
Q: How is Human coagulation factor VII concentrate BRP reconstituted for laboratory use?
A: The concentrate should be reconstituted using the provided buffer or sterile water. Add the solvent carefully and gently swirl until the lyophilized powder is fully dissolved, resulting in a neutral pH solution ready for testing.Q: What are the recommended storage conditions for this product to maintain stability?
A: Store the vials at 20C or below. When kept unopened and under these conditions, the concentrate maintains stability and usability for up to five years.Q: When is Human coagulation factor VII concentrate BRP typically used?
A: It is used in laboratory settings for coagulation studies, as a reference standard, and for calibration in factor VII assays in clinical diagnostics. It is not intended for direct clinical administration.Q: Where should Human coagulation factor VII concentrate BRP be handled?
A: This product is meant exclusively for use in laboratory environments by trained professionals, adhering to standard protocols for handling human-derived proteins.Q: What is the benefit of using this concentrate in diagnostic assays?
A: Using this high-purity, standardized reference enhances the accuracy and reliability of factor VII detection and quantification, improving the integrity of diagnostic results.Q: Does the product contain any known allergens?
A: Yes, as it is derived from human plasma, it contains human-derived proteins. Individuals should handle it according to appropriate safety protocols for biological materials.Q: Is Human coagulation factor VII concentrate BRP poisonous or hazardous under normal laboratory use?
A: No, it is non-poisonous when handled properly in controlled settings. However, as with all human-derived materials, standard laboratory biohazard precautions are necessary.

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Analytical Grade Chemicals Category
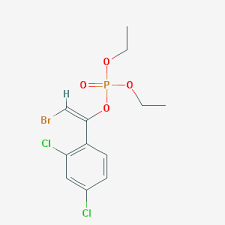
Bromfenvinphos- Ethyl
Price 100 INR / Kilograms
Minimum Order Quantity : 1 Kilograms
Molecular Weight : 404.02 Grams (g)
Molecular Formula : C12H14BrCl2O4P
Melting Point : 19 to 23�C
Purity : 98%
Gadolinium Standard for ICP
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Weight : 157.2 Grams (g)
Molecular Formula : Gd
Melting Point : 1,313 °C
Procyanidin C1
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Weight : 866.77 Grams (g)
Molecular Formula : C45H38O18
Purity : 98%
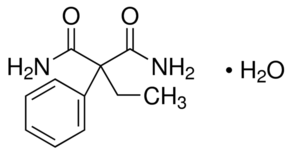
2-Ethyl-2-phenylmalonamide monohydrate
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Weight : 206.24 Grams (g)
Molecular Formula : C11H14N2O2
Melting Point : 109113°C
Purity : 95%

 Send Inquiry
Send Inquiry


 Send Inquiry
Send Inquiry Send SMS
Send SMS Call Me Free
Call Me Free