Human immunoglobulin BRP (ACA and Molecular size)
Human immunoglobulin BRP (ACA and Molecular size) Specification
- Usage
- For laboratory calibration standards, ACA, and chromatography
- Poisonous
- NO
- Grade
- Reference Standard
- Purity
- >98% by ACA
- Molecular Weight
- ~150 kDa (IgG)
- HS Code
- 30021090
- Properties
- High purity immunoglobulin suitable for ACA and molecular size analysis
- Shelf Life
- Refer to product label (typically 2 years when unopened at recommended conditions)
- Appearance
- White to off-white powder
- Physical Form
- Lyophilized
- Shape
- Powder
- Product Type
- Biological Reference Preparation (BRP)
- Smell
- Odorless
- Storage
- Store at 28C
- Ingredients
- Human immunoglobulin
- Solubility
- Soluble in water or physiological buffers
- Molecular Formula
- Not applicable (protein mixture)
- Application
- Analytical use: ACA (Agarose Cohn Absorption), molecular size calibration
- Intended Use
- For laboratory use only, not for human or animal therapeutic use
- Safety Information
- Refer to safety data sheet (SDS) before handling
- Packaging
- Supplied in sealed glass vials with desiccant
- Endotoxin Level
- <0.1 EU/mg protein
- Identification
- Confirmed by electrophoresis and immunochemical methods
- Code Number
- BRP batch (refer to product label)
- Handling Precautions
- Use appropriate aseptic technique, avoid repeated freeze/thaw cycles
- Protein Content
- Nominal concentration specified on label
- Stability
- Stable at recommended storage for shelf life
Human immunoglobulin BRP (ACA and Molecular size) Trade Information
- Main Domestic Market
- All India
About Human immunoglobulin BRP (ACA and Molecular size)
Human immunoglobulin BRP (ACA and Molecular size)
European Pharmacopoeia (EP) Reference Standard
Synonym: -Globulins human
CAS Number 9007-83-4
Properties
| Related Categories | Analytical Standards, Analytical/Chromatography, EP Standards, EP Standards G - H, Pharmacopeia & Metrological Institutes Standards |
| form | neat |
| format | neat |
Optimized for Analytical Calibration
This immunoglobulin reference standard offers outstanding purity and consistency, making it ideal for accurate calibration in ACA and molecular size analysis. Its lyophilized, odorless powder form dissolves easily in water or physiological buffers, facilitating effortless preparation for laboratory protocols. Each batch is thoroughly characterized and verified for identity, purity, and stability, ensuring dependable results every time.
Safe Handling and Stable Storage
Maintaining the integrity of your immunoglobulin standard is straightforward with its robust packaging and recommended storage at 28C. The desiccant and sealed vials protect against moisture, while the stability profile generally allows for a two-year shelf life when unopened. Always consult the safety data sheet (SDS), use aseptic techniques, and avoid repeated freeze/thaw cycles to ensure optimal performance and safety during use.
FAQs of Human immunoglobulin BRP (ACA and Molecular size):
Q: How should Human immunoglobulin BRP be stored for optimal stability?
A: It should be stored at 28C in its originally sealed glass vial with desiccant. Keeping it at recommended temperature conditions ensures stability and maintains its shelf life, typically up to two years as indicated on the product label.Q: What is the intended use of this reference standard?
A: The product is designed exclusively for laboratory applications, such as ACA (Agarose Cohn Absorption) and molecular size calibration. It must not be used for therapeutics or for administration to humans or animals.Q: When should the solution be prepared, and can it be refrozen?
A: Prepare the solution immediately before use, ensuring proper aseptic techniques. Repeated freeze/thaw cycles should be avoided to preserve the integrity and performance of the immunoglobulin.Q: Where can this product be sourced from in India?
A: It can be obtained through authorized laboratory suppliers and traders across India. Confirm the suppliers credentials to ensure you receive a genuine, batch-verified product.Q: What process is used for identification and quality confirmation?
A: Quality and identity are confirmed by electrophoresis and immunochemical assays, with a purity of over 98% determined by ACA. Endotoxin levels are controlled to below 0.1 EU/mg protein for reliable analytical use.Q: How is Human immunoglobulin BRP commonly used in the laboratory?
A: It serves as a reference standard for calibration in ACA procedures, molecular size determination, and chromatographic applicationshelping to ensure accuracy and reproducibility in quantification and analysis.Q: What are the main benefits of using this specific immunoglobulin reference standard?
A: Its high purity, stringent quality controls, and certified analytical properties make it a trusted choice for calibration and quality assurance in laboratory settings, delivering precise and reproducible results.
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
More Products in Analytical Grade Chemicals Category
Ginseng dry extract HRS
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Weight : 843 Grams (g)
Molecular Formula : C42H66O17
Purity : 99%
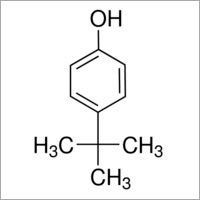
4-tert-Butylphenol
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Weight : 150.22 Grams (g)
Molecular Formula : C10H14O
Melting Point : 98 C
Purity : 98%
Hexahydro-4-methylphthalic anhydride, mixture of cis and trans
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Weight : 174.12 Grams (g)
Molecular Formula : C3H6N6O3
Melting Point : 29C
Purity : 7478%
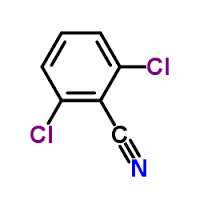
Dichlobenil
Price 100 INR
Minimum Order Quantity : 1 , , Kilograms
Molecular Weight : 172.01 Grams (g)
Molecular Formula : C7H3Cl2N
Melting Point : 144.5 C

 Send Inquiry
Send Inquiry



 Send Inquiry
Send Inquiry Send SMS
Send SMS