Human immunoglobulin for electrophoresis BRP
Human immunoglobulin for electrophoresis BRP Specification
- Refractive Rate
- Not determined
- Ph Level
- 7.0 8.0 (1% solution)
- Shape
- Amorphous
- Molecular Formula
- C6452H10092N1716O2004S40 (approximate, IgG)
- Physical Form
- Powder
- Shelf Life
- 36 months (unopened)
- Structural Formula
- Protein complex structure, typical for human IgG
- Poisonous
- NO
- Grade
- BRP (Biological Reference Preparation)
- Density
- Gram per cubic centimeter(g/cm3)
- Product Type
- Reference Standard
- Storage
- Store at 28C, protect from light
- Solubility
- Soluble in saline or buffer
- Appearance
- White to off-white powder
- Molecular Weight
- ~150 kDa
- Smell
- Odourless
- Properties
- For analytical electrophoresis
- Melting Point
- Not determined
- Purity
- >98% (Electrophoretic)
- Application
- Electrophoresis, standardization, laboratory reference
- Ingredients
- Human Immunoglobulin
- HS Code
- 3822
- Usage
- For calibration and validation of electrophoretic procedures
- Lot Number/Batch Number
- Refer to product packaging
- Allergen Status
- Source: Human plasma
- Handling Precautions
- Handle using appropriate laboratory safety protocols
- Shipping Conditions
- Shipped in cold chain (refrigerated)
- Microbial Status
- Sterility not guaranteed; intended for laboratory/analytical use only
- Reconstitution
- Use physiological saline or prescribed buffer for dissolution
- Supplier Name
- EDQM/European Pharmacopoeia
- Expiry Date
- Refer to product packaging
- Intended Use
- For pharmaceutical quality control and research use only, not for therapeutic use
- Packaging Size
- Standard vial
Human immunoglobulin for electrophoresis BRP Trade Information
- Main Domestic Market
- All India
About Human immunoglobulin for electrophoresis BRP
Human immunoglobulin for electrophoresis BRP
European Pharmacopoeia (EP) Reference Standard
Synonym: -Globulins human
CAS Number 9007-83-4
Properties
| Related Categories | Analytical Standards, Analytical/Chromatography, EP Standards, EP Standards G - H, Pharmacopeia & Metrological Institutes Standards |
| form | neat |
| format | neat |
Precision in Electrophoresis Standardization
Achieve reliable calibration and validation of your electrophoretic analyses with the Human Immunoglobulin BRP. This biological reference standard ensures accuracy and consistency in laboratory results, supporting regulatory compliance and robust quality control in pharmaceutical settings.
Safe Handling and Storage Protocols
Prioritize laboratory safety by handling this product according to established protocols. Store the powder at 28C away from direct light to preserve its stability and integrity. Always refer to lot and expiry details on the packaging for effective inventory management.
Optimized for Analytical Excellence
Specifically designed for analytical applications, this immunoglobulin reference offers high purity, reproducibility, and solubility in physiological buffers. Its use assists researchers and quality control professionals in maintaining stringent standards during electrophoresis and related assays.
FAQs of Human immunoglobulin for electrophoresis BRP:
Q: How should Human Immunoglobulin for Electrophoresis BRP be reconstituted before use?
A: Dissolve the powder using physiological saline or the prescribed buffer solution according to laboratory protocols specified for electrophoretic analysis.Q: What is the recommended storage condition for this product to maintain its quality?
A: Store the standard vial at refrigerated temperatures between 28C, and protect it from light. Adhering to these conditions helps preserve potency throughout its 36-month shelf life.Q: When should I refer to the lot number and expiry date information?
A: Always check the product packaging for the lot and expiry details before use to ensure you are working with valid and traceable reference material in your laboratory procedures.Q: Where is this product intended to be used?
A: It is intended for laboratory and analytical use only, specifically within pharmaceutical quality control and research environments. It must not be used therapeutically in humans or animals.Q: What processes benefit from using Human Immunoglobulin BRP?
A: This reference preparation is ideal for calibration and validation in electrophoretic procedures, ensuring precise and standardized results in analytical laboratories.Q: What precautions are necessary when handling this powder?
A: Handle the product using appropriate laboratory safety protocols, as sterility is not guaranteed and it originates from human plasma; avoid direct contact and follow standard operating procedures.Q: What are the key benefits of using this BRP in research or quality control?
A: Using this biological reference ensures reproducibility, accuracy, and regulatory alignment in analytical assays, supporting high-confidence decision making in pharmaceutical analysis.
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
More Products in Analytical Grade Chemicals Category
BDE No 15 solution
Molecular Weight : 552.7 g/mol
Molecular Formula : C12H9Br5O
Melting Point : 190205C
Purity : 98% minimum
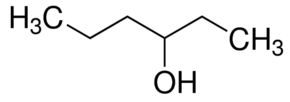
3-Hexanol
Price 100 INR
Minimum Order Quantity : 1 , , Kilograms
Molecular Weight : 102.174 Grams (g)
Molecular Formula : C6H14O
Purity : 99%
Zidovudine
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Weight : 267.242 Grams (g)
Molecular Formula : C10H13N5O4
Melting Point : 119121 C. (B567.99.w99)
Captan
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Weight : 300.6 Grams (g)
Molecular Formula : C9H8Cl3NO2S
Melting Point : 178 C
Purity : 97%

 Send Inquiry
Send Inquiry


 Send Inquiry
Send Inquiry Send SMS
Send SMS