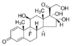
Hydroxypropyl cellulose
MOQ : 1 Kilograms
Hydroxypropyl cellulose Specification
- Molecular Formula
- C56H108O30
- Molecular Weight
- 806.9 Grams (g)
- Melting Point
- 100-150° C
- Appearance
- white to slightly yellow-colored, odorless and tasteless powder.
- Usage
- Lacrisert, manufactured by Aton Pharma, is a formulation of HPC used for artificial tears. It is used to treat medical conditions characterized by insufficient tear production such as keratoconjunctivitis sicca), recurrent corneal erosions, decreased corneal sensitivity, exposure and neuroparalytic keratitis.
Hydroxypropyl cellulose Trade Information
- Minimum Order Quantity
- 1 Kilograms
- Payment Terms
- Cash in Advance (CID), Cash Advance (CA)
- Supply Ability
- 500 Kilograms, KG Per Month
- Delivery Time
- 2-8 Week
- Sample Available
- Yes
- Sample Policy
- Sample costs shipping and taxes has to be paid by the buyer
- Packaging Details
- Carton and Poly Bag.
- Main Export Market(s)
- Western Europe, Eastern Europe, Central America, Africa, Middle East, South America, Asia, North America, Australia
- Main Domestic Market
- All India
About Hydroxypropyl cellulose
Hydroxypropyl celluloseUnited States Pharmacopeia (USP) Reference StandardCAS Number9004-64-2PropertiesRelated CategoriesAnalytical Standards,Analytical/Chromatography,Pharmacopeia & Metrological Institutes Standards,USP Standards,USP Standards G - Hform neatautoignition temp. 752Fsolubility DMF: solubleDMSO: solubleH2O: insoluble (hot)H2O: soluble (cold)alcohol: solublechloroform: solubledioxane: solubleisopropanol: solublemethanol: solublepropylene glycol: solubledensity 0.5g/mLat 25C(lit.)format neatEuropean Pharmacopoeia (EP) Reference StandardPropertiesRelated CategoriesAnalytical Standards,Analytical/Chromatography,EP Standards,EP Standards G - H,Pharmacopeia & Metrological Institutes Standardsautoignition temp. 752Fdensity 0.5g/mLat 25C(lit.)Pharmaceutical Secondary Standard; Certified Reference MaterialPropertiesRelated CategoriesAdditional Standards,Analytical Standards,Analytical/Chromatography,Chromatography,Pharmaceutical Secondary Standards,Pharmaceutical and Drug Standards,Pharmacopeia & Metrological Institutes Standards,Secondary Pharmaceutical Standardspharmaceutical secondary standardautoignition temp. 752Fpackaging pkg of 1gdensity 0.5g/mLat 25C(lit.)DescriptionAnalysis NoteThese secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.General descriptionPharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.FAQs of Hydroxypropyl cellulose:
Q: What is the molecular weight of Hydroxypropyl cellulose?
A: The molecular weight of Hydroxypropyl cellulose is 806.9 grams (g).Q: What is the melting point range of Hydroxypropyl cellulose?
A: The melting point of Hydroxypropyl cellulose ranges between 100-150C.Q: What does Hydroxypropyl cellulose look like?
A: Hydroxypropyl cellulose appears as a white to slightly yellow-colored, odorless and tasteless powder.Q: What is the molecular formula of Hydroxypropyl cellulose?
A: The molecular formula of Hydroxypropyl cellulose is C56H108O30.Q: What are some medical conditions treated with Hydroxypropyl cellulose?
A: Hydroxypropyl cellulose, in formulations like Lacrisert manufactured by Aton Pharma, is used to treat conditions such as keratoconjunctivitis sicca, recurrent corneal erosions, decreased corneal sensitivity, exposure keratitis, and neuroparalytic keratitis.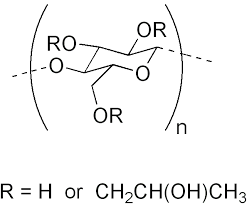
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
More Products in Analytical Grade Chemicals Category
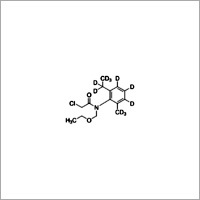
Acetochlor-(2-ethyl-6-methylphenyl-d11)
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Weight : 269.77 Grams (g)
Molecular Formula : C14H20ClNO2
Purity : 80%
Melting Point : <0°C
Brombuterol hydrochloride
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Weight : 402.55 Grams (g)
Molecular Formula : C12H19Br2ClN2O
Purity : 98%
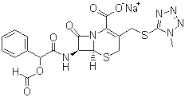
Cefamandole nafate
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Weight : 462.505
Molecular Formula : C19H18N6O6S2
Melting Point : (°C), 182184,
Triamcinolone
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Weight : 434.5 Grams (g)
Molecular Formula : C24H31FO6
Purity : 98%
Melting Point : 292° and 294°C.

 Send Inquiry
Send Inquiry


 Send Inquiry
Send Inquiry Send SMS
Send SMS