n-Butyl Lithium(2.5 M in Hexane)
Price 100 INR/ Piece
n-Butyl Lithium(2.5 M in Hexane) Specification
- Storage Instructions
- Store under inert atmosphere, in cool, dry place, tightly sealed
- Packaging Type
- Glass bottle, ampoule
- Size
- Custom packaging sizes available (standard: 100 mL, 250 mL, 500 mL)
- Molecular Weight
- 64.06 g/mol
- Boiling point
- Hexane solvent: 68.7 C
- Melting Point
- -70 C
- Shelf Life
- 12 months
- Flash Point
- -22 C
- Ph Level
- Strongly basic (pH unavailable for nonaqueous solution)
- Physical State
- Liquid
- Usage
- As a strong base and nucleophile in organic synthesis
- Purity
- 2.5 M Solution in Hexane
- Molecular Formula
- C4H9Li
- Density
- 0.68 Gram per cubic centimeter(g/cm3)
- CAS No
- 109-72-8
- Grade
- Reagent Grade
- Type
- Solution
- Application
- Organic synthesis, polymerization initiator, chemical reagent
- Appearance
- Colorless to pale yellow liquid
- Purity(%)
- 97%
n-Butyl Lithium(2.5 M in Hexane) Trade Information
- Minimum Order Quantity
- 1 Piece
- Payment Terms
- Cash in Advance (CID), Cash Advance (CA)
- Supply Ability
- 3 Pieces Per Week
- Delivery Time
- 3-4 Week
- Main Export Market(s)
- Australia, North America, South America, Middle East, Africa, Central America, Western Europe, Asia, Eastern Europe
- Main Domestic Market
- All India
- Certifications
- ISO 9001: 2015 CERTIFICATE
About n-Butyl Lithium(2.5 M in Hexane)
n-Butyllithium solution
2.5 M in hexanes
Synonym: n-BuLi, Butyl lithium, Butyllithium solution, Lithium-1-butanide-
CAS Number109-72-8
-
Linear Formula CH3(CH2)3Li
-
Molecular Weight 64.06
| Quality Level | 200 |
| form | liquid |
| concentration | 2.5 M in hexanes |
| density | 0.693 g/mL at 25 C |
| storage temp. | 2-8C |
Powerful Reagent for Synthetic Applications
n-Butyl Lithium (2.5 M in Hexane) stands out for its strong basicity and nucleophilicity, making it essential for various organic synthesis and polymerization reactions. Its reliable activity ensures efficient reaction initiation and substrate deprotonation, which is particularly useful in the pharmaceutical, academic, and industrial laboratories focused on advanced organic chemistry.
Safe Handling and Storage Guidelines
Due to its high reactivity and pyrophoric nature, n-Butyl Lithium must always be handled under an inert atmosphere, such as nitrogen or argon. The solution is supplied anhydrous, packed in sealed glass containers or ampoules to prevent exposure to air and moisture. Store in a cool, dry place, tightly sealed, and follow all safety protocols when transferring or using the reagent.
FAQ's of n-Butyl Lithium(2.5 M in Hexane):
Q: How should n-Butyl Lithium (2.5 M in Hexane) be handled for laboratory use?
A: Always handle n-Butyl Lithium under an inert atmosphere, such as nitrogen or argon, using appropriate personal protective equipment. Avoid contact with skin, eyes, and moisture to prevent hazardous reactions. Use only in a well-ventilated fume hood, and follow institutional safety guidelines.Q: What are the primary uses of n-Butyl Lithium in organic synthesis?
A: This reagent acts as a strong base and nucleophile in organic synthesis, enabling deprotonation, alkyl lithium compound preparation, and initiation of polymerization processes. It is widely employed in research, pharmaceuticals, and chemical manufacturing for these purposes.Q: When does the n-Butyl Lithium solution begin to lose effectiveness, and how can this be prevented?
A: n-Butyl Lithium typically has a shelf life of 12 months when stored properly under inert atmosphere and tightly sealed. Exposure to moisture, air, or prolonged storage at room temperature can degrade its effectiveness, so always store it according to label instructions.Q: Where should n-Butyl Lithium (2.5 M in Hexane) be stored to maintain stability?
A: Store the solution in a cool, dry place away from sources of ignition, air, and moisture. Keep containers sealed, under inert gas such as nitrogen, and in an area designated for pyrophoric materials. This prevents decomposition and autoignition.Q: What is the process for safe disposal of n-Butyl Lithium residue?
A: Dispose of residues by carefully quenching remaining n-Butyl Lithium under inert atmosphere with a suitable alcohol (such as isopropanol) in a controlled manner. Follow local regulations and institutional hazardous waste disposal policies.Q: What are the principal hazards associated with n-Butyl Lithium (2.5 M in Hexane)?
A: n-Butyl Lithium is classified as a flammable, pyrophoric liquid and is highly toxic if swallowed, inhaled, or in contact with skin. It can spontaneously ignite in air and reacts violently with water, acids, and oxidizing agents. Always prioritize safety.Q: What benefits does using n-Butyl Lithium offer for organic synthesis and research?
A: Its high purity, strong basicity, and nucleophilicity provide consistent results in various advanced syntheses, notably enabling efficient and selective reactions. This reagent supports demanding research and manufacturing needs in academia and industry.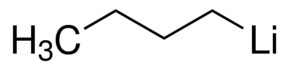

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Laboratory Chemicals Category
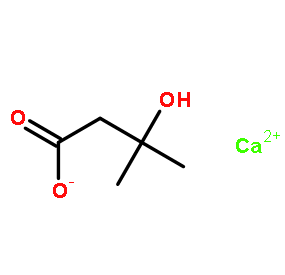
HMB Calcium
Price 100 INR / Kilograms
Minimum Order Quantity : 1 Kilograms
Appearance : White crystal powder
Purity(%) : 99%
Type : Other, Intermediates
CAS No : 135236725
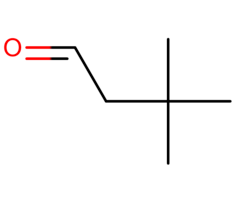
3,3-Dimethylbutyraldehyde liquid
Price 100 INR / Kilograms
Minimum Order Quantity : 1 Kilograms
Appearance : Colorless to light yellow liquid
Purity(%) : 98%
Type : Other, Intermediates
CAS No : 2987168
N-Heptane Chemical
Minimum Order Quantity : 10 Liters
Appearance : Liquid
Purity(%) : 99.99
Type : Industrial Lab Chemicals
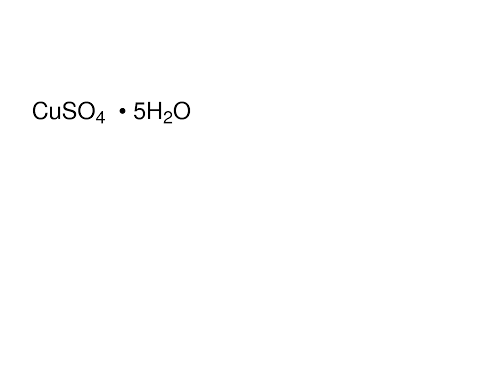
copper sulphate pentahydrate
Price 100 INR / Milliliter
Minimum Order Quantity : 1 Kilograms
Appearance : Blue Crystalline Powder
Purity(%) : 98%
Type : Copper Sulfate Pentahydrate, Other
CAS No : 7758998

 Send Inquiry
Send Inquiry


 Send Inquiry
Send Inquiry Send SMS
Send SMS Call Me Free
Call Me Free