Nitric Acid
MOQ : 1 Piece
Nitric Acid Specification
- CAS No
- 7697-37-2
- Grade
- ACS reagent
- Type
- Scientific Lab Chemicals
- Purity(%)
- 70%
Nitric Acid Trade Information
- Minimum Order Quantity
- 1 Piece
- FOB Port
- MUMBAI
- Payment Terms
- Cash in Advance (CID), Cash Advance (CA)
- Supply Ability
- 10 Pieces Per Week
- Delivery Time
- 3-4 Week
- Packaging Details
- Packaging 100 mL in glass bottle 2.2 L in poly bottle 2.5, 62.5 L in PVC-coated bottle 500, 6500 mL in PVC-coated bottle
- Main Export Market(s)
- Western Europe, Australia, North America, South America, Eastern Europe, Middle East, Central America, Africa, Asia
- Main Domestic Market
- All India
- Certifications
- ISO CERTIFICATE : 9001:2015
About Nitric Acid
Nitric acid ACS reagent, 70% General descriptionNitric acid (HNO3) is a highly corrosive mineral acid.It is usually utilized for abstracting transition metal catalyst from single-walled carbon nanotubes (SWNTs) in order to purify them.The effect of nitric acid on the oxidation of multiwalled carbon nanotubes (MWCNTs) has been investigated by sample weight, Raman spectrum, solubility, morphology and alignment examinations.ApplicationNitric acid was used to increase the number of acid sites by altering the surface of activated carbon.It may be used: In combination with acetic anhydride and zeolite catalysts for the preparation ofp-nitrotoluene with high regioselectivity.In the pretreatment of rice straw for enhanced production of xylose.To minimize the extent of cation ordering in LiNi0.5Mn1.5O4, a transition metal-substituted spinel material, by surface treatment. grade ACS reagent vapor pressure 8 mmHg ( 20 C) assay 68.0-70.0% (ACS specification) 70% ign. residue 5ppm color APHA: 10 bp 120.5C(lit.) density 1.413g/mLat 20C (lit.) anion traces chloride (Cl-): 0.5ppm sulfate (SO42-): 1ppm cation traces As: 0.01ppm Fe: 0.2ppm heavy metals (as Pb): 0.2ppm CAS Number 7697-37-2 Empirical Formula (Hill Notation)HNO3 Molecular Weight63.01FAQs of Nitric Acid:
Q: What is the chemical composition and purity of Nitric Acid offered?
A: The Nitric Acid offered has a purity of 70% and is classified as ACS reagent grade.Q: What is the CAS number for Nitric Acid?
A: The CAS number for Nitric Acid is 7697-37-2.Q: Is Nitric Acid suitable for scientific laboratory applications?
A: Yes, Nitric Acid is specifically designed as a Scientific Lab Chemical and meets ACS reagent grade standards.Q: Can Nitric Acid be used for analytical purposes?
A: Yes, as an ACS reagent grade chemical, Nitric Acid is suitable for analytical and laboratory applications.Q: What type of product classification does Nitric Acid fall under?
A: Nitric Acid is classified under Scientific Lab Chemicals.
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
More Products in Laboratory Chemicals Category
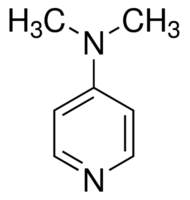
4-(Dimethylamino)Pyridine >99%
Price 100 INR / Piece
Minimum Order Quantity : 1 Piece
CAS No : 1122583
Type : Other, Chemical Compound
Purity(%) : >99%
Appearance : White to light yellow crystalline powder
J T Baker
CAS No : 64197
Type : Other, Acetic Acid
Purity(%) : 99.7%
Appearance : Clear, colorless liquid
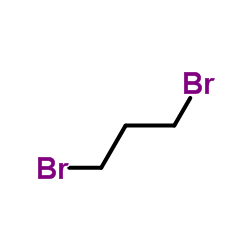
3-Bromo-1-propanol Liquid
Price 100 INR / Kilograms
Minimum Order Quantity : 1 Kilograms
CAS No : 627189
Type : Industrial Lab Chemicals
Purity(%) : 97%.
Appearance : Yellow liquid
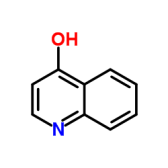
4-Hydroxyquinoline
Price 100 INR
Minimum Order Quantity : 1 Kilograms
CAS No : 611369
Type : Industrial Lab Chemicals
Purity(%) : 98%
Appearance : Buff to offwhite powder

 Send Inquiry
Send Inquiry



 Send Inquiry
Send Inquiry Send SMS
Send SMS Call Me Free
Call Me Free