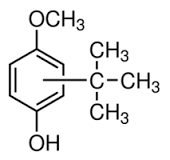
Pivmecillinam impurity C
MOQ : 1 Kilograms
Pivmecillinam impurity C Specification
- Molecular Weight
- 476 Grams (g)
- Molecular Formula
- C21H34ClN3O5S
- Usage
- Pivmecillinam is an antibiotic primarily used to treat bacterial infections of the urinary tract.
- Purity
- 98%
- Melting Point
- 119 °C
Pivmecillinam impurity C Trade Information
- Minimum Order Quantity
- 1 Kilograms
- Payment Terms
- Cash in Advance (CID), Cash Advance (CA)
- Supply Ability
- 500 Kilograms KG Per Month
- Delivery Time
- 2-8 Week
- Sample Available
- Yes
- Sample Policy
- Sample costs shipping and taxes has to be paid by the buyer
- Packaging Details
- Carton and Poly Bag.
- Main Export Market(s)
- Western Europe, Eastern Europe, Middle East, Africa, South America, Asia, Central America, North America, Australia
- Main Domestic Market
- All India
About Pivmecillinam impurity C
Pivmecillinam impurity C European Pharmacopoeia (EP) Reference Standard Synonym: (2RS,4S)-2-[[[(Hexahydro-1H-azepin-1-yl)methylene]amino]methyl]-5,5-dimethyl-4-thiazolidinecarboxylic acid (2,2-dimethyl-1-oxopropoxy)methyl ester Empirical Formula (Hill Notation) C20H35N3O4S Molecular Weight 413.57 Properties form neat format neat Description Packaging Unit quantity: 10 mg. Subject to change. The product is delivered as supplied by the issuing Pharmacopoeia. General description This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia .FAQs of Pivmecillinam impurity C:
Q: What is the primary application of Pivmecillinam impurity C?
A: Pivmecillinam impurity C is primarily used in the synthesis and study of Pivmecillinam, an antibiotic used to treat bacterial infections of the urinary tract.Q: What is the molecular formula of Pivmecillinam impurity C?
A: The molecular formula of Pivmecillinam impurity C is C21H34ClN3O5S.Q: What is the purity level of Pivmecillinam impurity C?
A: The purity level of Pivmecillinam impurity C is 98%.Q: What is the molecular weight of Pivmecillinam impurity C?
A: The molecular weight of Pivmecillinam impurity C is 476 grams (g).Q: What is the melting point of Pivmecillinam impurity C?
A: The melting point of Pivmecillinam impurity C is 119 C.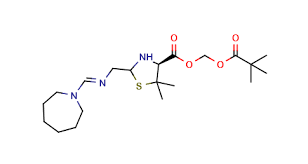
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
More Products in Analytical Grade Chemicals Category
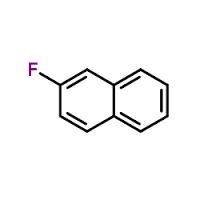
2- Fluoronaphthalene solution
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C10H7F
Molecular Weight : 146.164 Grams (g)
Purity : 98%
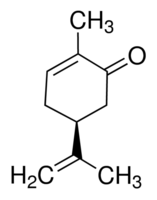
(+)-Carvone
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C10H14O
Molecular Weight : 150.22 Grams (g)
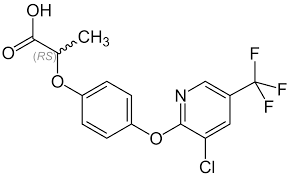
Haloxyfop-2-ethoxyethyl
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C19H19ClF3NO5
Molecular Weight : 433.8 Grams (g)
Purity : 99%
Melting Point : 5557°C
Butylated hydroxyanisole
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C11H16O2
Molecular Weight : 180.24 Grams (g)
Purity : 92%
Melting Point : 48 °C

 Send Inquiry
Send Inquiry


 Send Inquiry
Send Inquiry Send SMS
Send SMS