Xylose
MOQ : 1 Kilograms
Xylose Specification
- Usage
- Xylose is a sugar isolated from wood. D-Xylose is a sugar widely used as a diabetic sweetener in food and beverage. Xylose has also been used as a diagnostic agent to observe malabsorption. Reduction of xylose by catalytic hydrogenation produces the common food additive sweetener substitute xylitol Xylitol.
- Appearance
- monoclinic needles or prisms, colourless
- Molecular Formula
- C5H10O5
- Melting Point
- 144 to 145 °C (291 to 293 °F; 417 to 418 K)
- Molecular Weight
- 150.13 Grams (g)
Xylose Trade Information
- Minimum Order Quantity
- 1 Kilograms
- Payment Terms
- Cash Advance (CA), Cash in Advance (CID)
- Supply Ability
- 500 Kilograms KG Per Month
- Delivery Time
- 2-8 Week
- Sample Available
- Yes
- Sample Policy
- Sample costs shipping and taxes has to be paid by the buyer
- Packaging Details
- Carton and Poly Bag.
- Main Export Market(s)
- Western Europe, Australia, South America, Eastern Europe, Middle East, Central America, Africa, Asia, North America
- Main Domestic Market
- All India
About Xylose
Xylose is a naturally occurring sugar primarily extracted from wood. This versatile sugar exhibits a molecular formula of C5H10O5 and appears as colorless monoclinic needles or prisms. Xylose is widely recognized for its applications in food and beverage as a diabetic sweetener, offering a healthier alternative for individuals managing their glucose levels. It also plays a critical role as a diagnostic agent in assessing malabsorption conditions within the body. With a melting point of 144 to 145 C (291 to 293 F), Xylose showcases exceptional stability. Furthermore, its reduction through catalytic hydrogenation leads to the production of xylitol, a popular sweetener substitute in the food industry. Offering a molecular weight of 150.13 g, Xylose is sought after for its reliable properties, making it a preferred choice for industries focusing on food, beverages, and diagnostic applications.
FAQs of Xylose:
Q: What is Xylose primarily used for?
A: Xylose is primarily used as a diabetic sweetener in food and beverage applications and as a diagnostic agent for malabsorption studies.Q: What is the appearance of Xylose?
A: Xylose appears as colorless monoclinic needles or prisms.Q: What is the melting point of Xylose?
A: Xylose melts at 144 to 145 C (291 to 293 F).Q: How is Xylose converted to xylitol?
A: Xylose is reduced via catalytic hydrogenation to produce xylitol.Q: What is the molecular weight of Xylose?
A: Xylose has a molecular weight of 150.13 grams (g).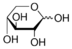
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
More Products in Analytical Grade Chemicals Category
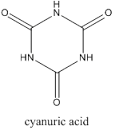
Cyanuric acid
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C3H3N3O3
Molecular Weight : 129.07 Grams (g)
Melting Point : 320 °C
Purity : 98%
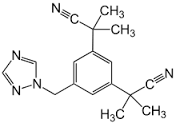
Anastrozole
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : ;C17H19N5
Molecular Weight : 293.4 Grams (g)
Melting Point : 137139°C
Purity : 98%
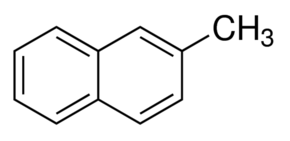
2- Methylnaphthalene
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C11H10
Molecular Weight : 142.2 Grams (g)
Purity : 97%
Glyceryl trioleate
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Formula : C57H104O6
Molecular Weight : 885.432 Grams (g)
Melting Point : 5 C; 41 F; 278 K

 Send Inquiry
Send Inquiry


 Send Inquiry
Send Inquiry Send SMS
Send SMS