Zinc (Zn) standard solution
Zinc (Zn) standard solution Specification
- Properties
- Precise concentration of Zinc ions in a liquid matrix with excellent stability for calibration use
- HS Code
- 38220090
- CAS No
- 7440-66-6 for elemental Zn or respective CAS for solution compounds
- Storage
- Store in a cool dry place away from direct sunlight; keep container tightly closed
- Melting Point
- N/A (Zinc metal melts at 419.5C but standard solution does not apply)
- Solubility
- Soluble in water
- Application
- Calibration standard in analytical chemistry
- Appearance
- Clear colorless liquid
- Poisonous
- NO
- Molecular Weight
- 65.38 g/mol
- Molecular Formula
- Zn
- Purity
- Defined concentration in parts per million (ppm) accurate to manufacturer specifications
- Smell
- Odorless
- Structural Formula
- Zinc ions (Zn) in aqueous solution
- Ph Level
- Variable depending on diluent typically neutral to slightly acidic
- Physical Form
- Liquid solution
- Density
- 1 Gram per cubic centimeter(g/cm3)
- Usage
- Used in atomic absorption spectroscopy (AAS) and other laboratory testing methods for trace-level Zinc quantification
- Shelf Life
- Stable for up to 12 months under proper storage conditions
- Shape
- N/A (liquid)
- Grade
- Analytical grade
- Ingredients
- Zinc compound dissolved in water or acid (e.g. zinc sulfate or zinc nitrate)
- EINECS No
- 231-175-3 for elemental Zn
About Zinc (Zn) standard solution
Zinc (Zn) Standard Solution is a high-purity analytical grade liquid designed for precise calibration in laboratory applications. This odorless, clear, and colorless solution contains a well-defined concentration of zinc ions in water or acid, such as zinc sulfate or zinc nitrate. With excellent stability, it is tailored for atomic absorption spectroscopy (AAS) and trace-level zinc quantification. It features solubility in water, variable pH levels (neutral to slightly acidic), and a density of 1 g/cm. The solution remains stable for up to 12 months under proper storage conditions: a cool, dry place, away from direct sunlight, with its container tightly closed. Non-toxic and odorless, it ensures safe handling while offering precise calibration for analytical chemistry and other laboratory testing methods. The molecular formula is Zn, with a molecular weight of 65.38 g/mol, adhering to HS Code 38220090 standards.
FAQs of Zinc (Zn) standard solution:
Q: What is the primary application of Zinc (Zn) Standard Solution?
A: It is primarily used as a calibration standard in analytical chemistry and laboratory tests, including atomic absorption spectroscopy (AAS) for trace-level zinc quantification.Q: How should Zinc (Zn) Standard Solution be stored?
A: Store it in a cool, dry place away from direct sunlight, ensuring the container is tightly sealed.Q: Is Zinc (Zn) Standard Solution poisonous?
A: No, it is non-toxic and safe for use in laboratories.Q: What is the shelf life of Zinc (Zn) Standard Solution?
A: It remains stable for up to 12 months under proper storage conditions.Q: What is the physical form and appearance of the solution?
A: The solution is liquid in form and appears as a clear, colorless liquid.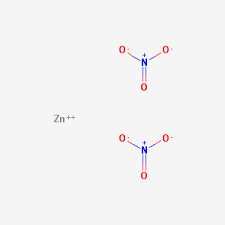
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
More Products in Analytical Grade Chemicals Category
Carvacrol
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Purity : 98%
Molecular Formula : C10H14O
Molecular Weight : 150.217 Grams (g)
Appearance : Colorless to yellow clear viscous liquid
Doxapram impurity B
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Purity : 99%
Molecular Formula : C22H28N2O2
Appearance : White Solid
Bacosine
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Purity : 99%
Molecular Formula : C30H48O3
Molecular Weight : 456.7 Grams (g)
Appearance : White powder
Bacopasaponin C
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Purity : 95%
Molecular Formula : C46H74O17
Molecular Weight : 899.1 Grams (g)
Appearance : White powder.

 Send Inquiry
Send Inquiry


 Send Inquiry
Send Inquiry Send SMS
Send SMS