2-Phenoxyethanol
2-Phenoxyethanol Specification
- Molecular Formula
- C8H10O2
- Molecular Weight
- 138.16 Grams (g)
- Physical Form
- Liquid
- Melting Point
- −2 °C (28 °F; 271 K)
- Appearance
- Colorless oily liquid
- Solubility
- Chloroform, Alkali, diethyl ether: soluble
2-Phenoxyethanol Trade Information
- Minimum Order Quantity
- 1 , , Kilograms
- Payment Terms
- Cash in Advance (CID), Cash Advance (CA)
- Supply Ability
- 500 Kilograms Per Month
- Delivery Time
- 3-4 Week
- Sample Available
- Yes
- Sample Policy
- Sample costs shipping and taxes has to be paid by the buyer
- Main Export Market(s)
- Western Europe, Australia, Middle East, Central America, South America, Asia, Eastern Europe, North America, Africa
- Main Domestic Market
- All India
About 2-Phenoxyethanol
2-Phenoxyethanol Analytical standard Synonym: Ethylene glycol monophenyl ether, PHE-G, PHE-S, PHE, PhG, Phenylglycol SDS Similar Products CAS Number 122-99-6 Linear Formula C6H5OCH2CH2OH Molecular Weight 138.16 grade analytical standard assay 99.5% (GC) form neat shelf life limited shelf life, expiry date on the label application(s) HPLC: suitable gas chromatography (GC): suitable refractive index n20/D 1.539 bp 244-246C mp 11-13C density 1.107g/mLat 20C(lit.) format neat analytical standard Synonym: Ethylene glycol monophenyl ether, PHE-G, PHE-S, PHE, PhG, Phenylglycol CAS Number 122-99-6 Linear Formula C6H5OCH2CH2OH Molecular Weight 138.16 grade analytical standard assay 99.5% (GC) form neat shelf life limited shelf life, expiry date on the label application(s) HPLC: suitable gas chromatography (GC): suitable refractive index n20/D 1.539 bp 244-246C mp 11-13C density 1.107g/mLat 20C(lit.) format neatFAQs of 2-Phenoxyethanol:
Q: What is the physical form of 2-Phenoxyethanol?
A: 2-Phenoxyethanol is available in liquid physical form.Q: What is the molecular formula of 2-Phenoxyethanol?
A: The molecular formula of 2-Phenoxyethanol is C8H10O2.Q: What is the melting point of 2-Phenoxyethanol?
A: The melting point of 2-Phenoxyethanol is -2 C (28 F; 271 K).Q: Can 2-Phenoxyethanol dissolve in chloroform and alkali?
A: Yes, 2-Phenoxyethanol is soluble in chloroform, alkali, and diethyl ether.Q: What is the appearance of 2-Phenoxyethanol?
A: 2-Phenoxyethanol appears as a colorless oily liquid.Q: What is the molecular weight of 2-Phenoxyethanol?
A: The molecular weight of 2-Phenoxyethanol is 138.16 Grams (g).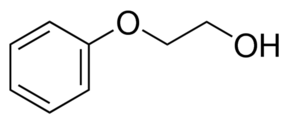

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Analytical Grade Chemicals Category
()-Miconazole Nitrate Salt
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Melting Point : 159 C
Molecular Weight : 479.1
Usage : Treat skin infections such as Athlete's foot, jock itch,
Molecular Formula : C18H15Cl4N3O4
Zearalenone solution
Price 100 INR / Kilograms
Minimum Order Quantity : 1 Kilograms
Melting Point : 167°C
Molecular Weight : 318.4 Grams (g)
Usage : Zearalenone solution may be used as analytical reference standard for the estimation of the analyte in biological samples and food products using various chromtography techniques.
Molecular Formula : C18H22O5
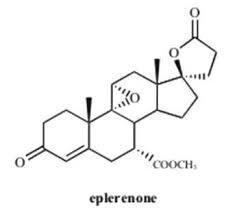
Eplerenone for peak identification
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Melting Point : >219°C (dec.)
Molecular Weight : 414.49 Grams (g)
Usage : Eplerenone is used alone or in combination with other medications to treat high blood pressure. Eplerenone is in a class of medications called mineralocorticoid receptor antagonists. It works by blocking the action of aldosterone, a natural substance in the body that raises blood pressure.
Zircaloy (C, N, O)
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Melting Point : 1850°C
Molecular Weight : 91.22 Grams (g)
Usage : Zircaloy 1 was developed as a replacement for existing tube bundles in submarine reactors in the 1950s, owing to a combination of strength, low neutron cross section and corrosion resistance. Zircaloy2 was inadvertently developed, by melting Zircaloy1 in a crucible previously used for stainless steel.
Molecular Formula : Zr

 Send Inquiry
Send Inquiry


 Send Inquiry
Send Inquiry Send SMS
Send SMS Call Me Free
Call Me Free