Ethylene oxide-13C2
MOQ : 1 Kilograms
Ethylene oxide-13C2 Specification
- Molecular Formula
- C2H4O
- Solubility
- Miscible
- Molecular Weight
- 44.05 Grams (g)
- Usage
- As a toxic gas that leaves no residue on items it contacts, ethylene oxide is a surface disinfectant that is widely used in hospitals and the medical equipment industry to replace steam in the sterilization of heat-sensitive tools and equipment, such as disposable plastic syringes.
- Density
- 882 Kilogram per cubic meter (kg/m3)
- Melting Point
- 112.46 °C (−170.43 °F; 160.69 K)
Ethylene oxide-13C2 Trade Information
- Minimum Order Quantity
- 1 Kilograms
- Payment Terms
- Cash Advance (CA), Cash in Advance (CID)
- Supply Ability
- 500 Kilograms, KG Per Month
- Delivery Time
- 2-8 Week
- Sample Available
- Yes
- Sample Policy
- Sample costs shipping and taxes has to be paid by the buyer
- Packaging Details
- Carton and Poly Bag.
- Main Export Market(s)
- Western Europe, Middle East, Central America, Eastern Europe, Africa, South America, Australia, Asia, North America
- Main Domestic Market
- All India
About Ethylene oxide-13C2
Ethylene oxide-13C299 atom %13C, 99% (CP), contains hydroquinone as stabilizerCAS Number84508-46-3Empirical Formula (Hill Notation)13C2H4OMolecular Weight46.04PropertiesRelated CategoriesAlphabetical Listings,Analytical Standards,Analytical/Chromatography,Chemical Synthesis,E-F,Fumigants,Insecticides,Pesticides & Metabolites Standards,Specialty Gases,Stable Isotopes,Synthetic ReagentsInChI Key IAYPIBMASNFSPL-ZDOIIHCHSA-Nisotopic purity 99atom %13Cassay 99% (CP)contains hydroquinone as stabilizerrefractive index n20/D1.3597(lit.)bp 10.7C(lit.)mp 111C(lit.)density 0.859g/mLat 25Cmass shift M+2FAQs of Ethylene oxide-13C2:
Q: What is the molecular formula of Ethylene oxide-13C2?
A: The molecular formula of Ethylene oxide-13C2 is C2H4O.Q: What is the molecular weight of Ethylene oxide-13C2?
A: The molecular weight of Ethylene oxide-13C2 is 44.05 grams (g).Q: What is the melting point of Ethylene oxide-13C2?
A: The melting point of Ethylene oxide-13C2 is 112.46 C (170.43 F; 160.69 K).Q: What is the density of Ethylene oxide-13C2?
A: The density of Ethylene oxide-13C2 is 882 kilograms per cubic meter (kg/m).Q: What is the usage of Ethylene oxide-13C2?
A: Ethylene oxide-13C2 is used as a toxic gas for surface disinfection, particularly in hospitals and the medical equipment industry, for sterilizing heat-sensitive tools and equipment like disposable plastic syringes.Q: What is the solubility of Ethylene oxide-13C2?
A: Ethylene oxide-13C2 is miscible.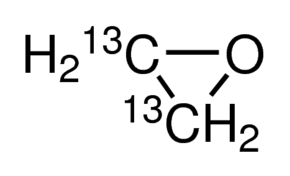
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
More Products in Analytical Grade Chemicals Category
Waste water (trace elements)
Molecular Weight : Varies depending on elements present
Molecular Formula : Composition varies based on trace elements
Purity : Depends on the level of contamination
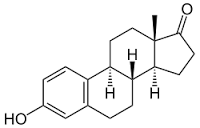
Estrone
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Weight : 270.366 Grams (g)
Molecular Formula : C18H22O2
Melting Point : 254.5
Purity : 97%
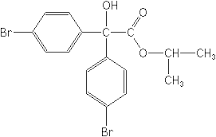
Bromopropylate (C17H16Br2O3)
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Weight : 428.1 Grams (g)
Molecular Formula : C17H16Br2O3
Melting Point : 77C
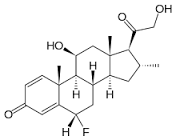
Fluocortolone pivalate
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Weight : 460.6 Grams (g)
Molecular Formula : C27H37FO5
Melting Point : (°C), 180182
Purity : 98%

 Send Inquiry
Send Inquiry



 Send Inquiry
Send Inquiry Send SMS
Send SMS