Human coagulation factor IX (rDNA)
Human coagulation factor IX (rDNA) Specification
- Density
- Gram per cubic centimeter(g/cm3)
- Purity
- >99%
- Grade
- Pharmaceutical Grade
- Physical Form
- Lyophilized Powder
- Taste
- Tasteless
- Molecular Formula
- C2339H3614N648O712S17 (approximate for protein backbone)
- Refractive Rate
- Not Determined
- Solubility
- Completely soluble in water (upon reconstitution)
- Ph Level
- 7.07.4 (reconstituted solution)
- Properties
- Highly purified recombinant protein, free from human plasma proteins, sterile
- Product Type
- Pharmaceutical Raw Material
- Usage
- Used in intravenous injection after reconstitution with sterile water
- Storage
- Store at 2C to 8C; protect from light
- HS Code
- 30021010
- CAS No
- 9002-08-0
- Ingredients
- Human coagulation factor IX (recombinant), stabilizers, buffer agents
- Shelf Life
- 2436 Months (unopened vial at recommended temperature)
- Melting Point
- Not applicable (lyophilized protein)
- Appearance
- White to off-white lyophilized cake or powder
- Structural Formula
- Recombinant protein structure analogous to human plasma-derived Factor IX
- Smell
- Odorless
- Shape
- Solid/Amorphous
- Poisonous
- Non-poisonous
- Molecular Weight
- ~55,000 Da
- Application
- Treatment and prevention of bleeding in patients with hemophilia B
- EINECS No
- 232-625-2
- Activity
- >250 IU/vial (varies depending on presentation)
- Virus Safety
- Viral inactivation/removal steps included during manufacturing
- Endotoxin Level
- <1.0 EU/IU
- Packaging
- Single use glass vial with diluent
- Administration Route
- Intravenous injection
- Allergen Information
- Free from known allergens
- Manufacturer Certification
- GMP, ISO certified
Human coagulation factor IX (rDNA) Trade Information
- Main Domestic Market
- All India
About Human coagulation factor IX (rDNA)
Human coagulation factor IX (rDNA)
European Pharmacopoeia (EP) Reference Standard
Properties
| Related Categories | Analytical Standards, Analytical/Chromatography, EP Standards, EP Standards G - H, Pharmacopeia & Metrological Institutes Standards |
Premium Quality and Safety Assured
This recombinant Factor IX is produced using advanced technology, resulting in a highly purified, plasma-free protein with over 99% purity. Each batch is rigorously tested for viral contaminants, endotoxin levels (<1.0 EU/IU), and allergenicity. Manufactured following GMP and ISO certifications, this product guarantees consistent quality and patient safety.
Easy Administration and Convenient Packaging
Each vial contains lyophilized powder along with a suitable diluent for reconstitution. It is designed for intravenous injection, ensuring rapid and effective delivery. The single-use glass vial packaging maintains sterility and product integrity, making it convenient for use in hospitals, clinics, or home-care settings.
FAQs of Human coagulation factor IX (rDNA):
Q: How is Human coagulation factor IX (rDNA) administered?
A: This product is administered intravenously after reconstitution with the provided sterile diluent. Healthcare professionals typically perform the injection to ensure proper dosage and technique.Q: What are the main benefits of using this recombinant Factor IX?
A: Key benefits include its high purity (>99%), absence of human plasma proteins, free-from-allergens formulation, and robust viral safety measures. These features reduce the risk of immune reactions and pathogen transmission.Q: When should Human coagulation factor IX (rDNA) be used?
A: It is used for the treatment and prevention of bleeding episodes in patients diagnosed with hemophilia B, particularly during surgeries, injury, or spontaneous bleeding events.Q: Where should this product be stored to maintain its effectiveness?
A: Store the unopened vial at 2C to 8C and protect it from light. Proper storage conditions help preserve the stability and efficacy of the product throughout its 2436 month shelf life.Q: What is the process for preparing this medication before administration?
A: Before use, reconstitute the lyophilized powder with the included sterile diluent according to the instructions. Gently mix until dissolved and use the solution immediately for intravenous injection.Q: Is Human coagulation factor IX (rDNA) suitable for individuals with allergies?
A: Yes, this recombinant product is free from known allergens, making it suitable for patients with common allergies or sensitivities to plasma-derived products.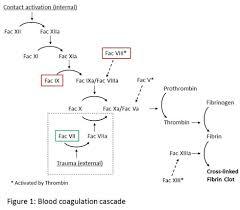
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
More Products in Analytical Grade Chemicals Category
Alumina powder for quantitative analysis by X-ray diffraction
Appearance : White fine powder
Molecular Weight : 101.96 g/mol
Purity : >99.9%
Molecular Formula : Al2O3
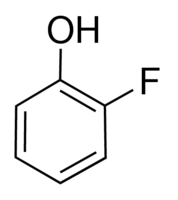
2-Fluorophenol
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Appearance : Light amber liquid
Molecular Weight : 112.1 Grams (g)
Purity : 98%
Molecular Formula : C6H5FO
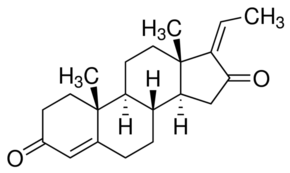
(Z)-Guggulsterone
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Appearance : White solid.
Molecular Weight : 312.4 Grams (g)
Purity : 96%
Molecular Formula : C21H28O2
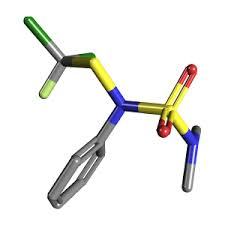
Dichlofluanid Solution
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Appearance : offwhite crystal
Molecular Weight : 333.2 Grams (g)
Purity : 98%
Molecular Formula : C9H11Cl2FN2O2S2

 Send Inquiry
Send Inquiry



 Send Inquiry
Send Inquiry Send SMS
Send SMS