Indapamide
Indapamide Specification
- Molecular Weight
- 749.7 Grams (g)
- Usage
- Indapamide tablets are indicated for the treatment of hypertension, alone or in combination with other antihypertensive drugs. Indapamide tablets are also indicated for the treatment of salt and fluid retention associated with congestive heart failure.
- Melting Point
- (°C), 160-162,
- Molecular Formula
- C32H34Cl2N6O7S2
- Appearance
- white to yellow-white crystalline
Indapamide Trade Information
- Minimum Order Quantity
- 1 Kiloampere
- Payment Terms
- Cash in Advance (CID), Cash Advance (CA)
- Supply Ability
- 500 Kiloamperes KG Per Month
- Delivery Time
- 2-8 Week
- Sample Available
- Yes
- Sample Policy
- Sample costs shipping and taxes has to be paid by the buyer
- Packaging Details
- Carton and Poly Bag.
- Main Export Market(s)
- Western Europe, Australia, Eastern Europe, Central America, Africa, Middle East, South America, Asia, North America
- Main Domestic Market
- All India
About Indapamide
Indapamideanalytical standard, for drug analysisSynonym: N-(4-Chloro-3-sulfamoylbenzamido)-2-methylindolineCAS Number26807-65-8Empirical Formula (Hill Notation)C16H16ClN3O3SMolecular Weight365.83PropertiesRelated CategoriesAdditional Standards,All Doping Standards (A-Z),Analytical Standards,Analytical/Chromatography,Cardiac Drug Standards,Chemical Structure,Chromatography,Diuretic,Drugs & Metabolites,Drugs of Abuse,Forensic and Veterinary Standards,Neat Compounds,Other Chemical Structure,Pharmaceuticals, Illicit Drugs & Alcohol,Steroids / Doping Agents Standardsgrade analytical standard, for drug analysisInChI Key NDDAHWYSQHTHNT-UHFFFAOYSA-Nform neatapplication(s) HPLC: suitablegas chromatography (GC): suitableformat neatUnited States Pharmacopeia (USP) Reference StandardPropertiesRelated CategoriesAnalytical Standards,Analytical/Chromatography,Pharmacopeia & Metrological Institutes Standards,USP Standards,USP Standards I - KInChI Key NDDAHWYSQHTHNT-UHFFFAOYSA-Nform neatformat neatEuropean Pharmacopoeia (EP) Reference StandardPropertiesRelated CategoriesAnalytical Standards,Analytical/Chromatography,EP Standards,EP Standards I - K,Pharmacopeia & Metrological Institutes StandardsInChI Key NDDAHWYSQHTHNT-UHFFFAOYSA-Nform neatformat neatFAQs of Indapamide:
Q: What is the molecular formula of Indapamide?
A: The molecular formula of Indapamide is C32H34Cl2N6O7S2.Q: What is the primary usage of Indapamide tablets?
A: Indapamide tablets are indicated for the treatment of hypertension, either alone or in combination with other antihypertensive drugs.Q: Can Indapamide tablets be used for congestive heart failure?
A: Yes, Indapamide tablets are indicated for the treatment of salt and fluid retention associated with congestive heart failure.Q: What is the melting point range of Indapamide?
A: The melting point range of Indapamide is 160-162C.Q: What does Indapamides appearance look like?
A: Indapamide appears as a white to yellow-white crystalline substance.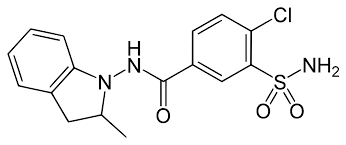

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Analytical Grade Chemicals Category
Hydrogen Halides/Halogens in Impinger Solution
Molecular Weight : Varies based on halogen (e.g. HF: 20.01 g/mol HCl: 36.46 g/mol)
Molecular Formula : HX (where X is a halogen: F Cl Br or I)
Purity : Dependent on preparation often >99% for analytical grades
Appearance : Clear or slightly cloudy liquid
Bis(methylthio)methane
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Weight : 108.23
Molecular Formula : C3H8S2
Purity : 99%
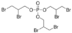
Tris(2,3-dibromopropyl) phosphate
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Weight : 697.6108 Grams (g)
Molecular Formula : C9H15Br6PO4
Purity : 98%
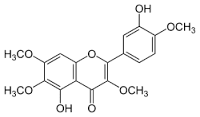
Casticin
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Weight : 374.3 Grams (g)
Molecular Formula : C19H18O8
Purity : 98%
Appearance : Yellow solid

 Send Inquiry
Send Inquiry


 Send Inquiry
Send Inquiry Send SMS
Send SMS