Granisetron impurity B
MOQ : 1 Kilograms
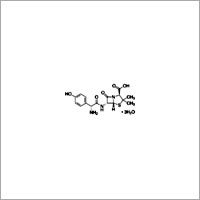
Granisetron impurity B Specification
- Molecular Weight
- 348.9 Grams (g)
- Appearance
- white to off-white solid
- Molecular Formula
- C18H25ClN4O
- Usage
- This medication is used alone or with other medications to prevent nausea and vomiting caused by cancer drug treatment (chemotherapy). It is also used to prevent and treat nausea and vomiting after surgery in adults. Granisetron works by blocking one of the body's natural substances (serotonin) that can cause vomiting.
- Melting Point
- (°C), 219 °C
Granisetron impurity B Trade Information
- Minimum Order Quantity
- 1 Kilograms
- Payment Terms
- Cash in Advance (CID), Cash Advance (CA)
- Supply Ability
- 500 Kilograms KG Per Month
- Delivery Time
- 2-8 Week
- Sample Available
- Yes
- Sample Policy
- Sample costs shipping and taxes has to be paid by the buyer
- Packaging Details
- Carton and Poly Bag.
- Main Export Market(s)
- Western Europe, North America, Eastern Europe, Africa, Middle East, South America, Asia, Central America, Australia
- Main Domestic Market
- All India
About Granisetron impurity B
Granisetron impurity BEuropean Pharmacopoeia (EP) Reference StandardSynonym: N-[(1R,3r,5S)-9-Methyl-9-azabicyclo[3.3.1]non-3-yl]-1H-indazole-3-carboxamideCAS Number107007-95-4Empirical Formula (Hill Notation)C17H22N4OMolecular Weight298.38PropertiesRelated CategoriesAnalytical Standards,Analytical/Chromatography,EP Standards,EP Standards G - H,Pharmacopeia & Metrological Institutes Standardsform neatformat neatFAQs of Granisetron impurity B:
Q: What is the appearance of Granisetron impurity B?
A: Granisetron impurity B appears as a white to off-white solid.Q: What is the usage of Granisetron impurity B?
A: Granisetron impurity B is used alone or with other medications to prevent nausea and vomiting caused by cancer drug treatment (chemotherapy). It is also used to prevent and treat nausea and vomiting after surgery in adults.Q: What is the molecular formula of Granisetron impurity B?
A: The molecular formula of Granisetron impurity B is C18H25ClN4O.Q: What is the molecular weight of Granisetron impurity B?
A: The molecular weight of Granisetron impurity B is 348.9 grams (g).Q: What is the melting point of Granisetron impurity B?
A: The melting point of Granisetron impurity B is 219 C.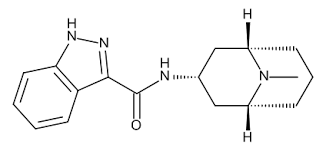
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
More Products in Analytical Grade Chemicals Category
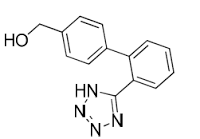
Irbesartan impurity A
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Weight : 446.54 Grams (g)
Purity : 98%
Melting Point : 180184C
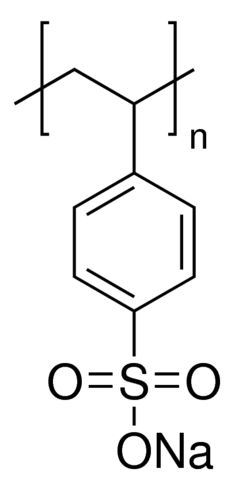
Poly(styrenesulfonic acid sodium salt)
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Weight : ~75,000
Purity : 98%
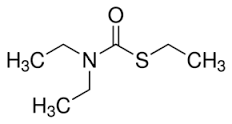
Ethiolat
Price 100 INR
Minimum Order Quantity : 1 Kilograms
Molecular Weight : 161.27 Grams (g)
Purity : 98%
Melting Point : 260 °C (500 °F; 533 K)
Molecular Formula : C7H15NOS
Amoxicillin trihydrate
Price 100 INR
Minimum Order Quantity : 1 , , Kilograms
Molecular Weight : 419.5 Grams (g)
Purity : 95%
Melting Point : >200°C
Molecular Formula : C16H25N3O8S

 Send Inquiry
Send Inquiry


 Send Inquiry
Send Inquiry Send SMS
Send SMS Call Me Free
Call Me Free